α-Truxillic Acid (Synonyms: Gratissimic Acid) |
| Catalog No.GC45224 |
α-Truxillic acid can be formed by the dimerization of two molecules of α-trans-cinnamic acid.
Products are for research use only. Not for human use. We do not sell to patients.
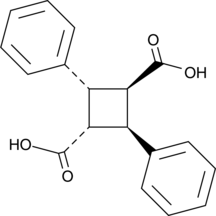
Cas No.: 490-20-0
Sample solution is provided at 25 µL, 10mM.
α-Truxillic acid can be formed by the dimerization of two molecules of α-trans-cinnamic acid. [1] It is related to incarvillateine, a natural antinociceptive compound derived from the Asian herb I. sinensis. [2] α-Truxillic acid and some of its derivatives significantly block inflammatory pain while having little effect on neurogenic pain, as indicated by the formalin test in mice. [2] [3] Related compounds, like SB-FI-26 , bind fatty acid binding protein 5 (FABP5). [4] This may be related to pain suppression, since FABP5 acts as a transporter of the endocannabinoid anandamide. [5] While certain derivatives of α-truxillic acid can directly activate peroxisome proliferator-activated receptor γ, α-truxillic acid has no such activity. [6]
Reference:
[1]. Benedict, J.B., and Coppens, P. Kinetics of the single-crystal to single-crystal two-photon photodimerization of α-trans-cinnamic acid to α-truxillic acid. J.Phys.Chem.A. 113(13), 3116-3120 (2009).
[2]. Chi, Y.M., Nakamura, M., Yoshizawa, T., et al. Anti-inflammatory activities of α-truxillic acid derivatives and their monomer components. Biological and Pharmaceutical Bullentin 28(9), 1776-1778 (2005).
[3]. Chi, Y.M., Nakamura, M., Zhao, X.Y., et al. Antinociceptive activities of α-truxillic acid and β-truxillic acid derivatives. Biological and Pharmaceutical Bullentin 29(3), 580-584 (2006).
[4]. Berger, W.T., Ralph, B.P., Kaczocha, M., et al. Targeting fatty acid binding protein (FABP) anandamide transporters - a novel strategy for development of anti-inflammatory and anti-nociceptive drugs. PLoS One 7(12), (2012).
[5]. Kaczocha, M., Glaser, S.T., and Deutsch, D.G. Identification of intracellular carriers for the endocannabinoid anandamide. Proceedings of the National Academy of Sciences of the United States of America 106(15), 6375-6380 (2009).
[6]. Steri, R., Rupp, M., Proschak, E., et al. Truxillic acid derivatives act as peroxisome proliferator-activated receptor γ activators. Bioorganic & Medicinal Chemistry Letters 20(9), 2920-2923 (2010).
Average Rating: 5 (Based on Reviews and 12 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















