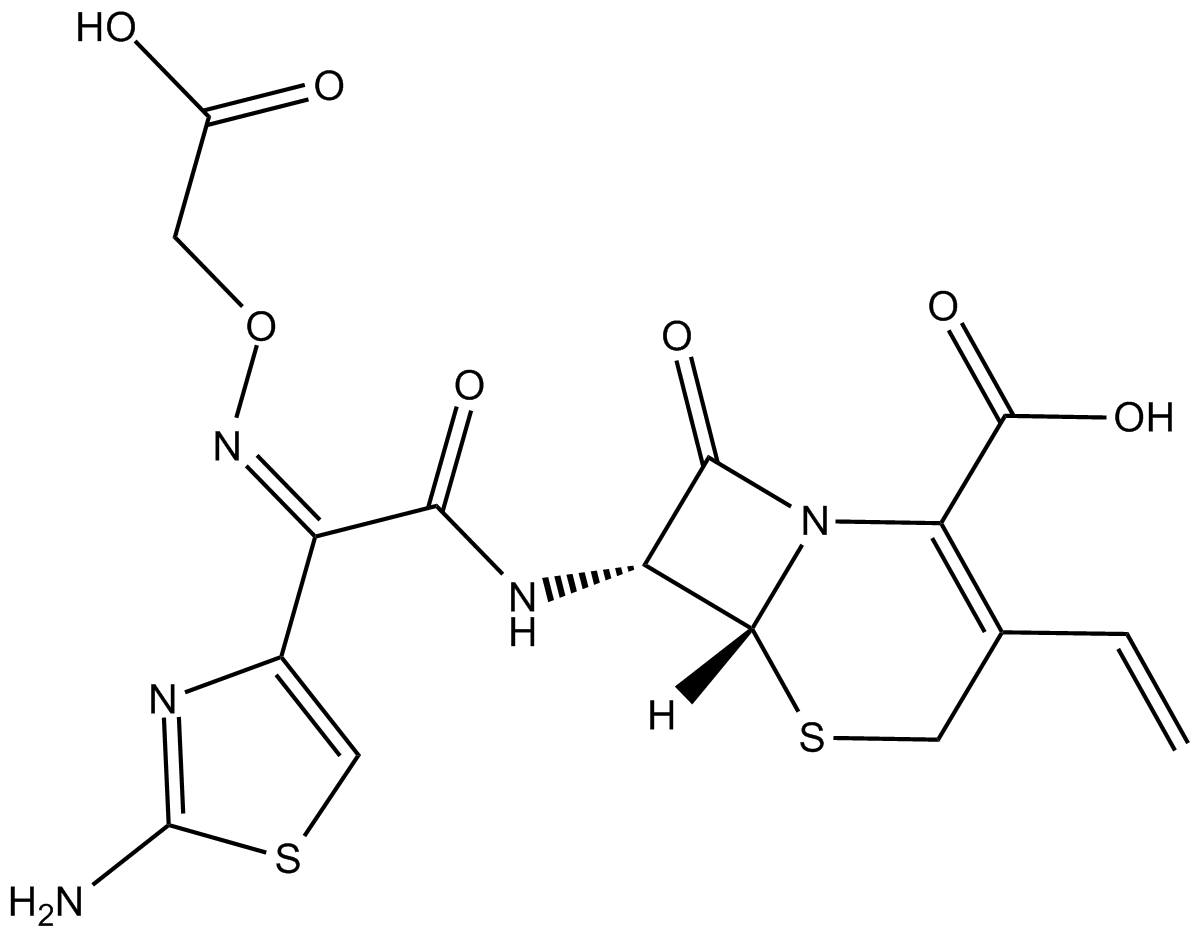
Cefixime (Synonyms: CL-284635,FK-027,FR17027) |
| Catalog No.GC14341 |
third generation cephalosporin antibiotic
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 79350-37-1
Sample solution is provided at 25 µL, 10mM.
MIC: from <0.025 to 25 μg/ml for E. coli, K. pneumoniae, and H. influenzae
Cefixime is a third generation cephalosporin antibiotic.
The cephalosporins, a class of β-lactam antibiotics, are originally derived from the fungus Acremonium.
In vitro: Previous study found that cefixime was more active than cephalexin, cefaclor, and amoxicillin against various gram-negative bacteria. Cefixime was also significantly more active than tested reference drugs against clinical isolates of Klebsiella pneumoniae, Escherichia coli, indole-positive and -negative Proteus species, Providencia species, and Neisseria gonorrhoeae. Moreover, cefixime was active against strains of K. pneumoniae, E. coli, as well as Proteus mirabilis resistant to the reference agents [1].
In vivo: The therapeutic activities of cefixime in mice infected with gram-negative bacilli were found to be far superior to the activities of cephalexin, cefaclor, and amoxicillin, but they were inferior to the activities against infection with Staphylococcus aureus [1].
Clinical trial: Previous study showed that the clinical success was observed in 94% of cefixime-treated patients. At the end of treatment, the overall eradication rate in the cefixime treatment group was 92% and ranged from 76% (cefaclor) to 98% (cefuroxime axetil) in the comparator treatment groups [2].
References:
[1] Kamimura, T. ,Kojo, H.,Matsumoto, Y., et al. In vitro and in vivo antibacterial properties of FK 027, a new orally active cephem antibiotic. Antimicrobial Agents and Chemotherapy 25(1), 98-104 (1984).
[2] Quintiliani R. Cefixime in the treatment of patients with lower respiratory tract infections: results of US clinical trials. Clin Ther. 1996 May-Jun;18(3):373-90; discussion 372.
Average Rating: 5 (Based on Reviews and 37 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















