Colcemid (Synonyms: Demecolcine, NSC 3096) |
| Catalog No.GC40664 |
Colcemid is a cytoskeletal inhibitor that induces mitotic arrest in the G2/M phase or meiotic arrest in the vesicle rupture (GVBD) phase in mammalian cells or oocytes, respectively.
Products are for research use only. Not for human use. We do not sell to patients.
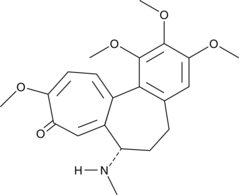
Cas No.: 477-30-5
Sample solution is provided at 25 µL, 10mM.
Colcemid is a cytoskeletal inhibitor that induces mitotic arrest in the G2/M phase or meiotic arrest in the vesicle rupture (GVBD) phase in mammalian cells or oocytes, respectively[1][2].Colchicine interferes with microtubule polymerization by tightly binding to tubulin dimers and prevents spindle microtubule formation by depolymerization [7].
Cell transformation was observed with doses which were non-cytotoxic and did not cause mitotic inhibition of the cells. Higher dose of colcemid (greater than 0.1 µg/ml) resulted in mitotic inhibition of the cells and a significant loss of colony forming ability, but no increase in the frequency of morphological transformation.A 14-fold increase in the number of aneuploid cells with a near diploid chromosome complement was found in cultures treated with 0.1 µg/ml colcemid and both chromosome loss and gain were induced[4]. Colcemid was more cytotoxic to cells in G2 + M than to G1 + S phase cells, and it slowed the progression of G1 cells to S. These effects of colcemid were much greater in aneuploid B16 melanoma cells than in pseudodiploid Chinese hamster ovary (CHO) cells[5]. Colcemid promotes UVC-induced apoptosis in Chinese hamster ovary cells (CHO.K1).Although colcemid did not affect the excision of UV-induced DNA damages such as photoproducts or cyclobutane pyrimidine dimers, colcemid accumulated the DNA breaks when it was added to cells following UV-irradiation[3].
A mitotic linear accumulation was obtained by continuous colcemid infusion at 5.82 µg/hr. Low dose colcemid infusion (0.582 and 1.455 µg/hr) for 14 hours did not accumulated mitotic cells, but doses more than 5.82 µg/hr of colcemid blocked it completely, accumulating 25.5% of cells after a 20 hours infusion[6].
References:
[1]. Tsuchida T, Yoshimura K, et,al. Colcemid-induced apoptosis of cultured human glioma: electron microscopic and confocal laser microscopic observation of cells sorted in different phases of cell cycle. Cytometry. 1998 Apr 1;31(4):295-9. doi: 10.1002/(sici)1097-0320(19980401)31:43.0.co;2-i. PMID: 9551605.
[2]. Ashley M Rozario, Sam DuwÉ, et,al.Ultra-Low Colcemid Doses Induce Microtubule Dysfunction as Revealed by Super-Resolution Microscopy bioRxiv 2020.08.13.249664; doi:https://doi.org/10.1101/2020.08.13.249664
[3]. Li H, Chang TW, et,al. Colcemid inhibits the rejoining of the nucleotide excision repair of UVC-induced DNA damages in Chinese hamster ovary cells. Mutat Res. 2005 Dec 30;588(2):118-28. doi: 10.1016/j.mrgentox.2005.09.005. Epub 2005 Nov 11. PMID: 16290038.
[4]. Tsutsui T, Maizumi H, et,al. Colcemid-induced neoplastic transformation and aneuploidy in Syrian hamster embryo cells. Carcinogenesis. 1984 Jan;5(1):89-93. doi: 10.1093/carcin/5.1.89. PMID: 6690091.
[5]. Bhuyan BK, Adams EG, et,al. Colcemid effects on B16 melanoma cell progression and aberrant mitotic division. J Cell Physiol. 1987 Aug;132(2):237-45. doi: 10.1002/jcp.1041320207. PMID: 3624316.
[6]. Nomura T. [In vivo cell cycle synchronization of the murine sarcoma 180 by continuous colcemid infusion (author's transl)]. Nihon Seikeigeka Gakkai Zasshi. 1980 Dec;54(12):1719-32. Japanese. PMID: 7288228.
[7]. Rieder CL, Palazzo RE. Colcemid and the mitotic cycle. J Cell Sci. 1992 Jul;102 ( Pt 3):387-92. doi: 10.1242/jcs.102.3.387. PMID: 1506421.
Average Rating: 5 (Based on Reviews and 29 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















