Erythromycin lactobionate |
| Catalog No.GC43627 |
A macrolide antibiotic
Products are for research use only. Not for human use. We do not sell to patients.
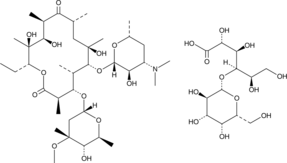
Cas No.: 3847-29-8
Sample solution is provided at 25 µL, 10mM.
Erythromycin is a macrolide antibiotic that inhibits bacterial protein synthesis by targeting the 50S ribosomal subunit, blocking the progression of nascent polypeptide chains.[1] It is effective against a host of bacterial genera, including Streptococcus, Staphylococcus, and Haemophilus (MIC90s range from 0.015-2.0 mg/l).[2] Erythromycin is known to potently inhibit the cytochrome P450 isoform CYP3A4, which can affect the metabolism of numerous clinically relevant medications.[3],[4] Erythromycin lactobionate is a soluble salt of erythromycin that is typically used for intraperitoneal or intravenous injections.[5],[6]
Reference:
[1]. Wilson, D.N. The A-Z of bacterial translation inhibitors. Crit. Rev. Biochem. Mol. Biol. 44(6), 393-433 (2009).
[2]. Kanatani, M.S., and Guglielmo, B.J. The new macrolides. Azithromycin and clarithromycin. Western J. Med. 160(1), 31-37 (1994).
[3]. Westphal, J.F. Macrolide - induced clinically relevant drug interactions with cytochrome P-450A (CYP) 3A4: An update focused on clarithromycin, azithromycin and dirithromycin. Br. J. Clin. Pharmacol. 50(4), 285-295 (2000).
[4]. Bibi, Z. Role of cytochrome P450 in drug interactions. Nutr. Metab. (Lond) 5(27), 1-10 (2008).
[5]. Hirakata, Y., Kaku, M., Tomono, K., et al. Efficacy of erythromycin lactobionate for treating Pseudomonas aeruginosa bacteremia in mice. Antimicrobial Agents and Chemotherapy 36(6), 1198-1203 (1992).
[6]. Austin, K.L., Mather, L.E., Philpot, C.R., et al. Intersubject and dose-related variability after intravenous administration of erythromycin. British Journal of Clinical Pharmacology 10(3), 273-279 (1980).
Average Rating: 5 (Based on Reviews and 21 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















