KD-3010 |
| Catalog No.GC31497 |
KD-3010 is a potent, orally active, and selective PPARδ agonist.
Products are for research use only. Not for human use. We do not sell to patients.
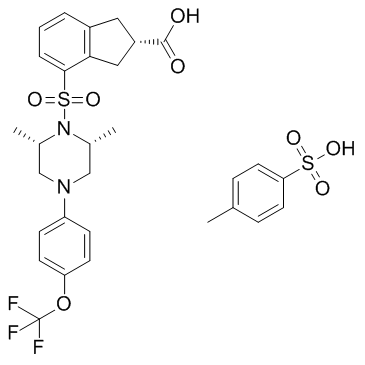
Cas No.: 934760-92-6
Sample solution is provided at 25 µL, 10mM.
KD3010 is a potent, orally active, and selective PPARδ agonist.
To determine whether PPARδ agonists are beneficial in experimental liver fibrosis, mice are treated orally with a PPARδ agonist, KD3010, or with the well-validated PPARδ agonist GW501516. KD3010, but not GW501516, shows hepatoprotective and antifibrotic effects in liver fibrosis induced by carbon tetrachloride (CCl4) or bile duct ligation (BDL). Liver injury is induced by repeated injections of CCl4, and mice are treated daily with vehicle, the widely used PPARδ agonist GW501516, or the PPARδ agonist KD3010 by oral gavage. Control oil-injected mice do not show any liver damage. Liver injury consisting of hepatocyte death and inflammation is seen in the vehicle- or GW501516-treated group injected with CCl4 on H&E-stained liver sections but is markedly reduced in the KD3010-treated group[1].
[1]. Iwaisako K, et al. Protection from liver fibrosis by a peroxisome proliferator-activated receptor δ agonist. Proc Natl Acad Sci U S A. 2012 May 22;109(21):E1369-76.
Average Rating: 5 (Based on Reviews and 24 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















