KU 59403 |
| Catalog No.GC33404 |
KU 59403 is a potent ATM inhibitor, with IC50 values of 3 nM, 9.1 μM and 10 μM for ATM, DNA-PK and PI3K, respectively.
Products are for research use only. Not for human use. We do not sell to patients.
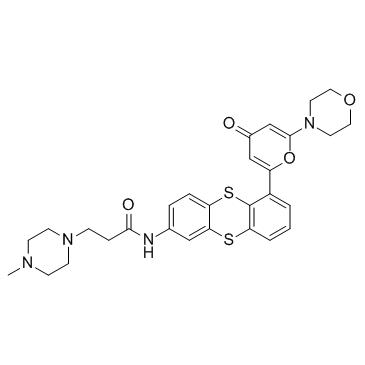
Cas No.: 845932-30-1
Sample solution is provided at 25 µL, 10mM.
KU 59403 is a potent ATM inhibitor, with an IC50 of 3 nM.
KU 59403 is a novel ATM inhibitor developed from LY294002, which is more potent against ATM than the previous lead KU 55933 (IC50=3 nM vs 13 nM), and has at least 1000 times greater specificity for ATM over other members of the PI3K family tested. KU 59403 is an effective chemosensitiser at a concentration of 1 μM. KU 59403 alone is not significantly cytotoxic to LoVo or SW620 cells (88±7% and 91±6% survival, respectively) but it enhances camptothecin cytotoxicity in both cell lines with greater enhancement being observed in the LoVo compared to the SW620 cells. KU 59403 also significantly enhances the cytotoxicity of fixed concentrations of etoposide (0.1 and 1 μM) or doxorubicin (10 or 100 nM) in these cell lines, with greater enhancement of etoposide in SW620 cells and of doxorubicin in LoVo cells. KU 59403 (1 μM) enhances etoposide (1 μM) cytotoxicity to a similar extent in HCT116 and HCT116-N7 cells, and in the p53 mutant SW620 cells and human breast cancer cell line, MDAMB-231, sensitisation is 11.9±4.7 and 3.8±1.8-fold respectively. Inhibition of IR-induced ATM activity by KU 59403 (1 μM) is approximately 50% in MDA-MB231 cells and >50% in HCT116 cells that have low ATM expression and activity[1].
Tumours in control mice reach 4 times their starting volume (RTV4) at a median time of 6.5 days. Treatment with etopophos alone causes a modest tumour growth delay of 4 days (time to RTV4=10.5 days). This delay is extended to 8.5 days when given with KU 59403 at 12.5 mg/kg i.p. twice daily for 5 days and 11.5 days (time to RTV4=18 days) when given with KU 59403 at 25 mg/kg i.p. twice daily for 5 days. This latter treatment is the most effective dosing schedule for KU 59403 identified; increasing etopophos efficacy by 190%. In contrast, when KU59403 is administered 4 hours after etopophos administration there is no increase in efficacy compared with etopophos alone. In the above studies, neither KU 59403 nor etopophos given as a single agent caused any measurable toxicity and the combination of drugs did not cause unacceptable toxicity. However, the etopophos-induced tumour growth delay is extended to 8.5 days by co-administration of KU 59403, representing a 300% enhancement of etopophos activity that was statistically significant. Toxicity, as measured by body weight loss, is tolerable and transient. In this study irinotecan alone causes an initial modest regression of the SW620 tumour followed by rapid re-growth resulting in a tumour growth delay of 7.5 days, which is extended to 19.5 days by the co-administration of KU 59403. As is clearly indicated from these data, enhancement of the efficacy of etopophos can be obtained in the SW620 and HCT116 xenograft models, and of irinotecan in the SW620 model, by combination with KU 59403 with little enhancement of toxicity[1].
[1]. Batey MA, et al. Preclinical evaluation of a novel ATM inhibitor, KU59403, in vitro and in vivo in p53 functional and dysfunctional models of human cancer. Mol Cancer Ther. 2013 Jun;12(6):959-67.
Average Rating: 5 (Based on Reviews and 31 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















