Lipoxin A4 (Synonyms: LXA4, 5(S),6(R),15(S)-TriHETE) |
| Catalog No.GC18552 |
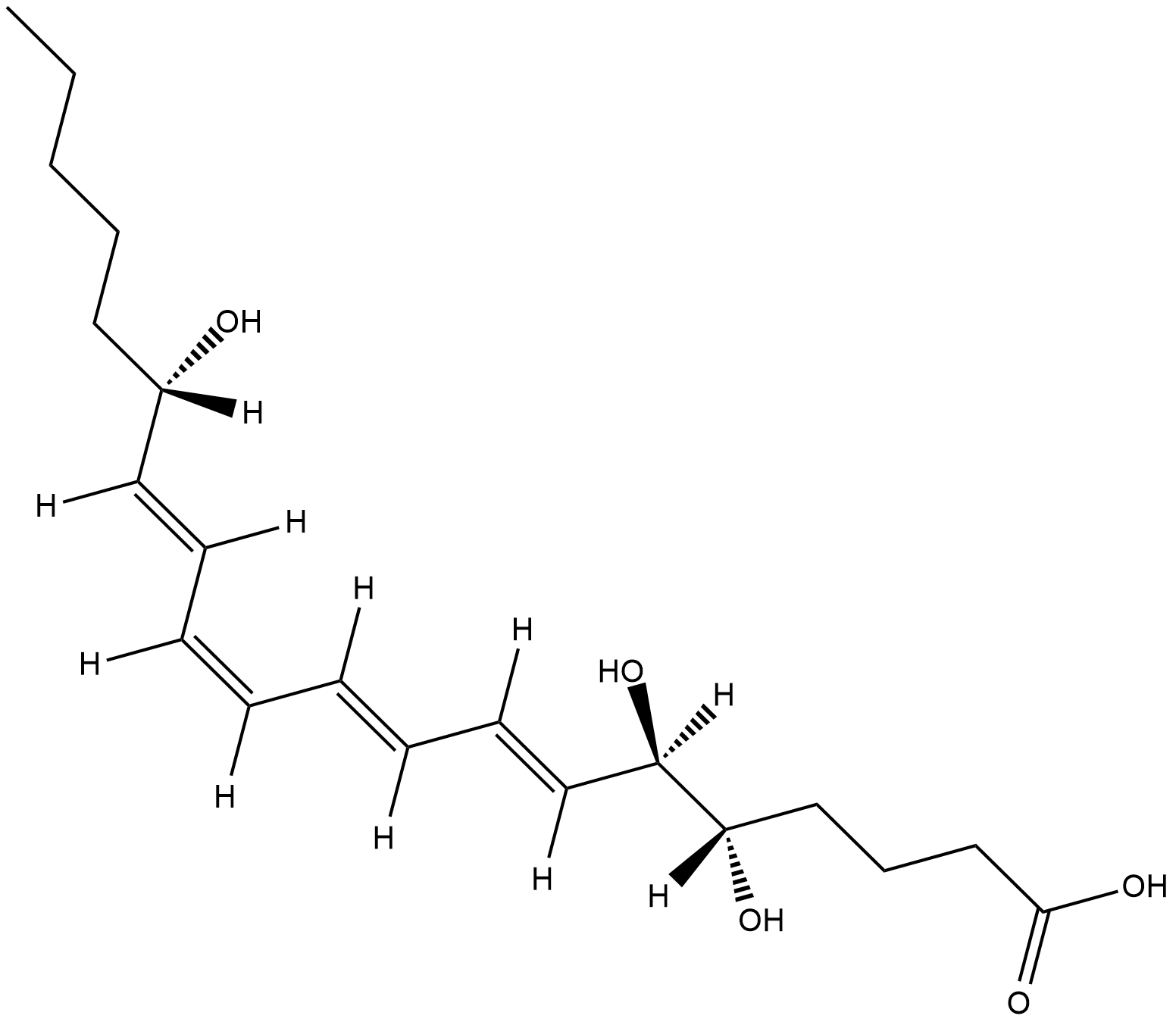
A trihydroxy fatty acid containing a conjugated tetraene
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 89663-86-5
Sample solution is provided at 25 µL, 10mM.
Lipoxin A4 (LXA4) is a trihydroxy fatty acid containing a conjugated tetraene, produced by the metabolism of (±)15-HETE or 15-HpETE with human leukocytes.[1] LXA4 is equipotent to leukotriene B4 in inducing superoxide generation in human neutrophils at 0.1 µM.2 LXA4 is associated with several other biological functions including leukocyte activation, chemotaxis effects, natural killer cell inhibition, and monocyte migration and adhesion.[2],[3],[4] Analytical and biological comparisons of synthetic LXA4 with endogenously derived LXA4 have confirmed its identity as matching the natural product.[5]
Reference:
[1]. Serhan, C.N., Nicolaou, K.C., Webber, S.E., et al. Lipoxin A. Stereochemistry and biosynthesis. J. Biol. Chem. 261(35), 16340-16345 (1986).
[2]. Serhan, C.N., Hamberg, M., and Samuelsson, B. Lipoxins: Novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc. Natl. Acad. Sci. U.S.A. 81(17), 5335-5339 (1984).
[3]. Ramstedt, U., Serhan, C.N., Nicolaou, K.C., et al. Lipoxin A-induced inhibition of human natural killer cell cytotoxicity: Studies on stereospecificity of inhibition and mode of action. J. Immunol. 138(1), 266-270 (1987).
[4]. Maddox, J.F., and Serhan, C.N. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: Selective inactivation by dehydrogenation and reduction. J. Exp. Med. 183(1), 137-146 (1996).
[5]. Serhan, C. . (2007).
Average Rating: 5 (Based on Reviews and 20 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




