MGCD-265 |
| Catalog No.GC13598 |
MGCD-265 is a potent and oral active inhibitor of c-Met and VEGFR2 tyrosine kinases, with IC50s of 29 nM and 10 nM, respectively. MGCD-265 has significant antitumor activity.
Products are for research use only. Not for human use. We do not sell to patients.
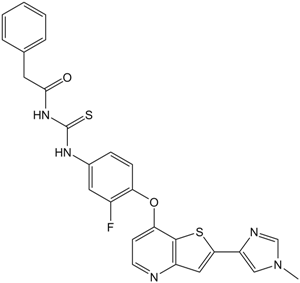
Cas No.: 875337-44-3
Sample solution is provided at 25 µL, 10mM.
MGCD-265 is a multi-target and ATP-competitive inhibitor of c-Met and VEGFR1, 2, 3 with IC50 values of 1 nM, 3 nM, 3 nM and 4 nM, respectively. [1]
In Non-small cell lung cancer xenograft models including one that harbored the TKI resistant EGFR mutation T790M, MGCD265 combined with either paclitaxel, docetaxel or erlotinib. Each combination elicited greater tumor response than agent alone, and displayed antiangiogenic properties with docetaxel [2].
MGCD265 has been studied in a variety of advanced solid tumors including NSCLC, as a monotherapy and in combination with either docetaxel or erlotinib. In a phase I study, MGCD265 was given orally from 24 mg/m2 daily to 235 mg/m2 twice daily uninterrupted to patients with advanced solid malignancy until disease progression [3]. In a phase I standard 3+3 dose escalation, MGCD265 (96 mg/m2 once daily up to 162 mg/m2 bid) was combined with erlotinib at 100 or 150 mg daily to determine safety, and 45 patients have been enrolled. In a separate phase I dose escalating trial, MGCD265 was combined with docetaxel (50 then 75 mg/m2 iv once every 3 weeks) in advanced solid tumors (n=34), including 9 NSCLC patients. The MTD of the microionized formulation of MGCD265 is 72 mg/m2 bid and docetaxel 75 mg/m2 every 3 weeks but has not been reached with the new formulation. [4][5]
References:
[1] Bonfils C. et al. AACR 2012 Annual Meeting, 2012. Abstract 1790.
[2]. Besterman JM, Fournel M, Dupont I, et al. Potent preclinical antitumor activity of MGCD265, an oral Met/VEGFR kinase inhibitor in phase II clinical development, in combination with taxanes or erlotinib. J Clin Oncol 2010;28:abstr e13595.
[3]. Kollmannsberger CK, Hurwitz H, Vlahovic G, et al. Phase I study of daily administration of MGCD265 to patients with advanced malignancies (Study 265-101). J Clin Oncol 2009;27:abstr e14525.
[4]. Kollmannsberger CK, Hurwitz H, Cleary JM, et al. MGCD265, a multitargeted oral tyrosine kinase receptor inhibitor of Met and VEGFR: Dose-escalation phase I study (abstr 3039). 2012 ASCO Annual Meeting Chicago, IL. J Clin Oncol 2012;30:abstr 3039.
[5]. Drouin MA, Kollmannsberger CK, Uronis HE, et al. Daily administration of MGCD265 to patients with solid tumors in a dose-escalation phase I study (study 265-101). J Clin Oncol 2010;28:abstr 3106.
Average Rating: 5 (Based on Reviews and 24 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















