IDO
Indoleamine 2,3-dioxygenase (IDO) is an enzyme catalyzing the oxidative degradation of L-tryptophan in the kynurenine pathway, in which the pyrrole ring of L-tryptophan is cleaved to generate N-formyl-kynurenine. Mature human IDO enzyme is a 45 kDa monomeric protein of 403 amino acids that is encoded by the IDO gene (15 kb with 10 exons). According to X-ray crystallographic analysis, the chemical structure of human IDO enzyme consists of two distinct α-helical domains (one small and one large) and a heme prosthetic group, where the heme is coordinated to the active site by a histidine (His) imidazole as the proximal fifth ligand.
Products for IDO
- Cat.No. Product Name Information
-
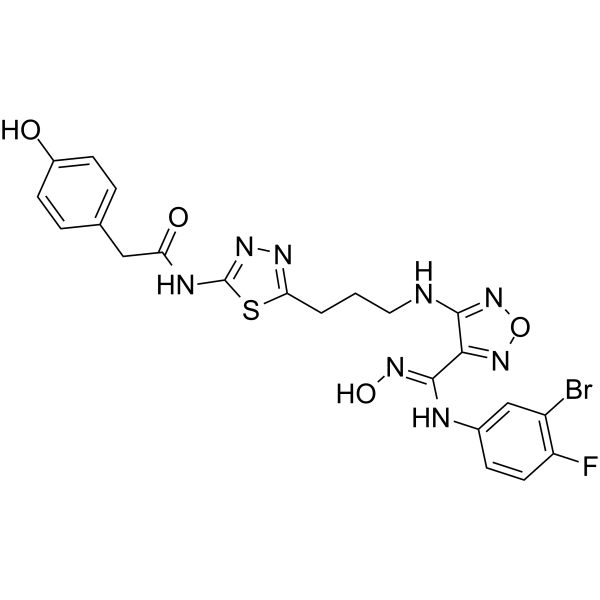
GC61750
(Rac)-Indoximod
(Rac)-Indoximod (1-Methyl-DL-tryptophan) is an indoleamine 2,3-dioxygenase (IDO) inhibitor.
-
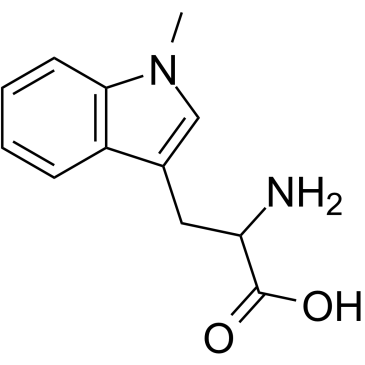
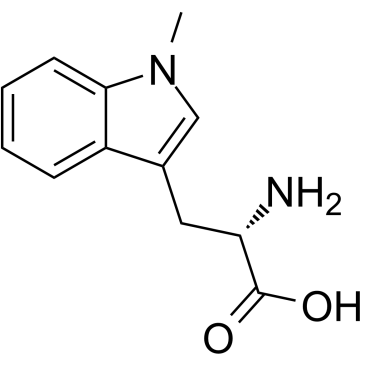
GC60417
(S)-Indoximod
(S)-Indoximod (1-Methyl-L-tryptophan) is an inhibitor of indoleamine 2,3-dioxygenase (IDO). (S)-Indoximod can be used for the research of cancer.
-
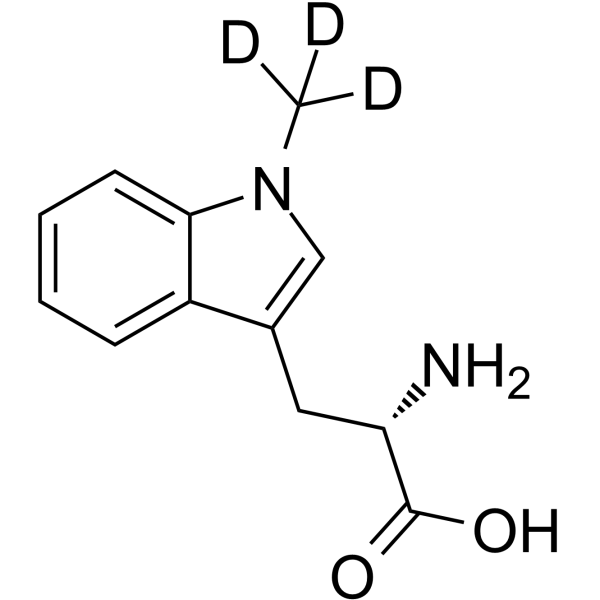
GC69891
(S)-Indoximod-d3
(S)-Indoximod-d3 is the deuterated form of (S)-Indoximod. (S)-Indoximod (1-Methyl-L-tryptophan) is an inhibitor of indoleamine 2,3-dioxygenase (IDO). (S)-Indoximod can be used for cancer research.
-
GC19079
BMS-986205
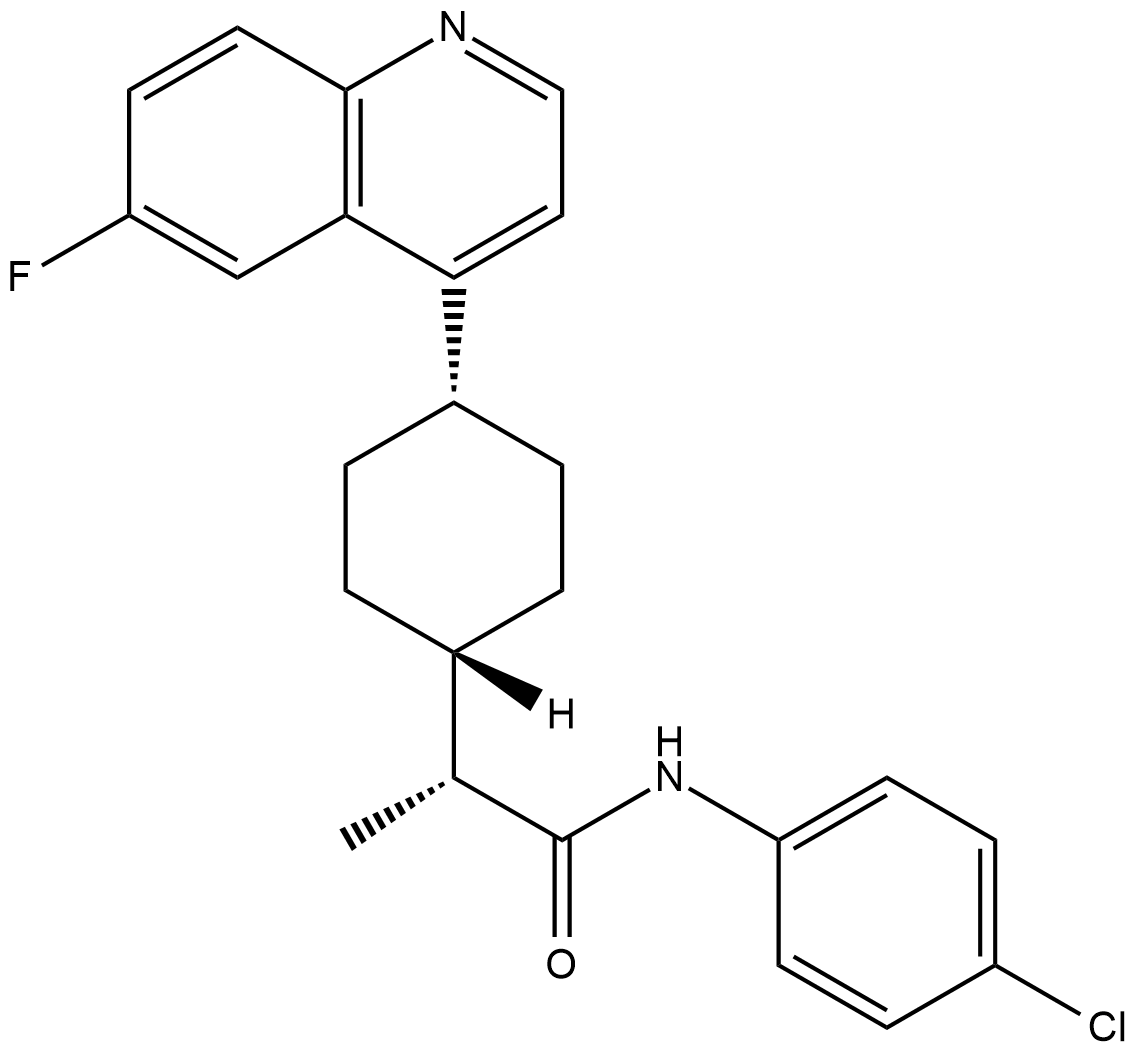
BMS-986205 (BMS-986205) is a selective and irreversible indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor with an IC50 value of 1.1 nM in IDO1-HEK293 cells. BMS-986205 is well tolerated with potent pharmacodynamic activity in advanced cancers.
-
GC62496
BMS-986242
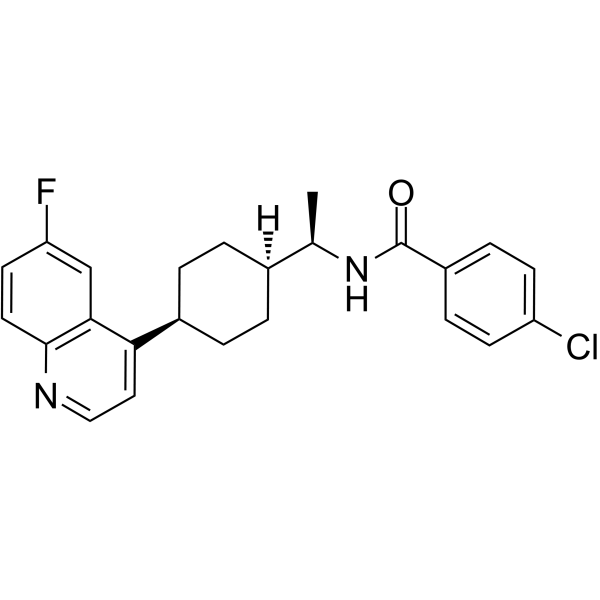
BMS-986242 is an orally active, potent and selective indoleamine-2,3-dioxygenase 1 (IDO1) inhibitor. BMS-986242 can be used for the research of cancer.
-
GN10414
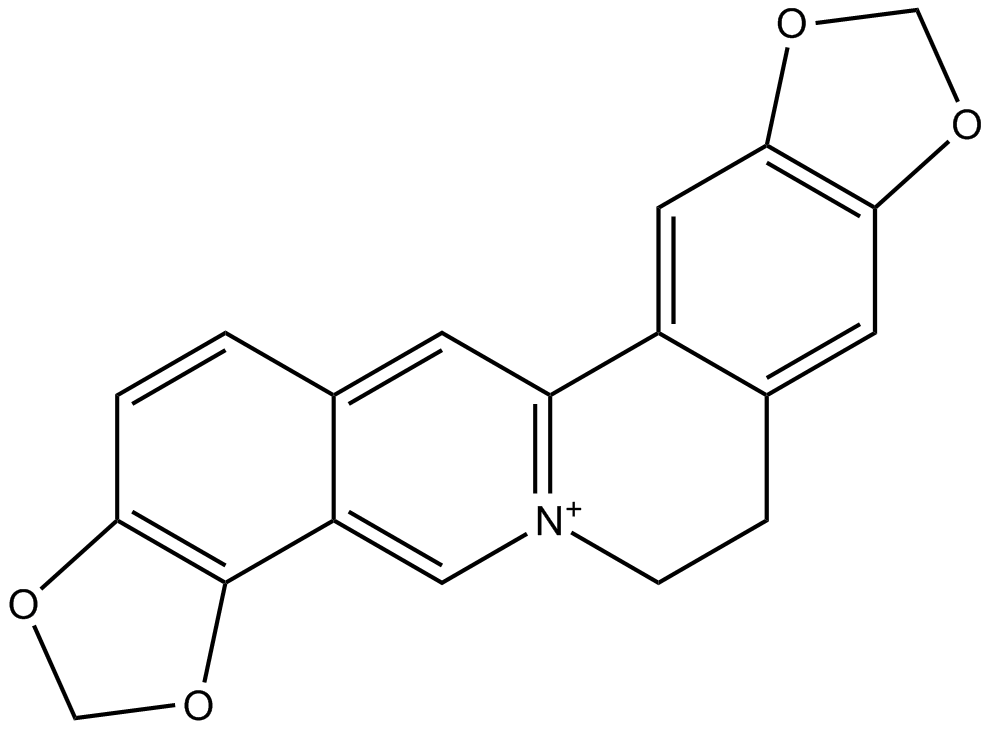
Coptisine
-
GN10577
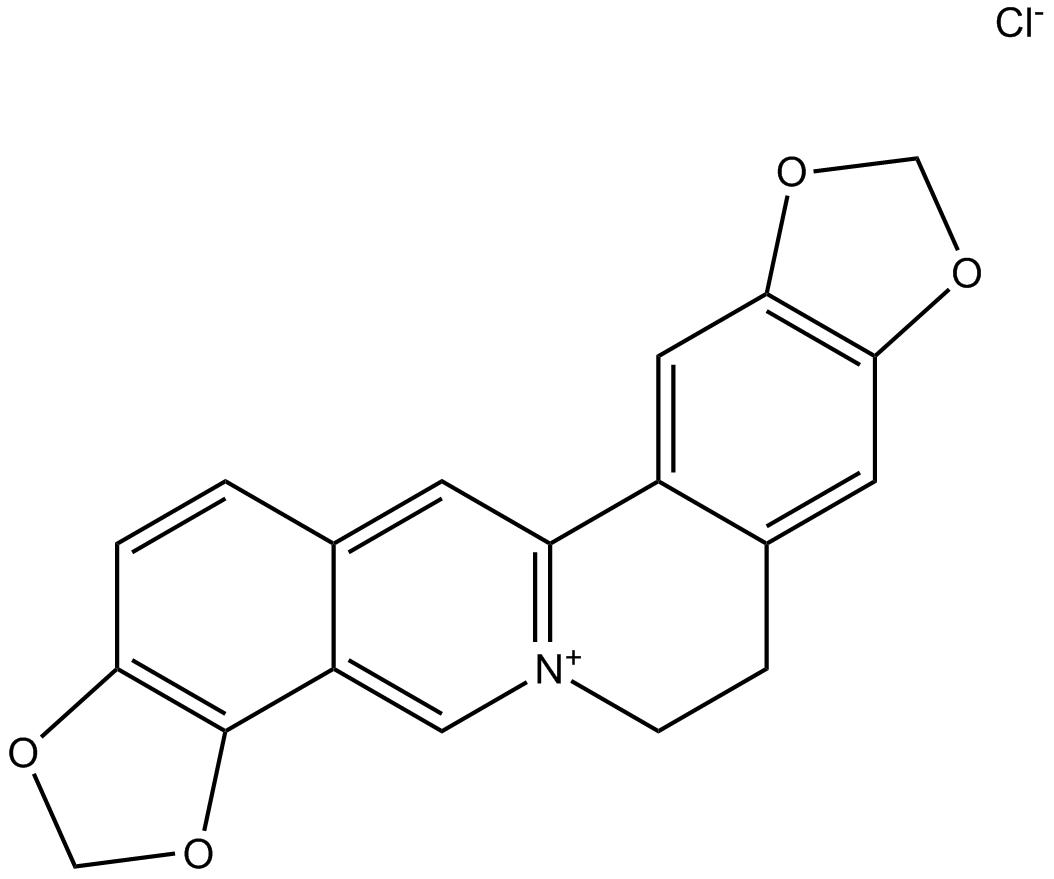
Coptisine chloride
-
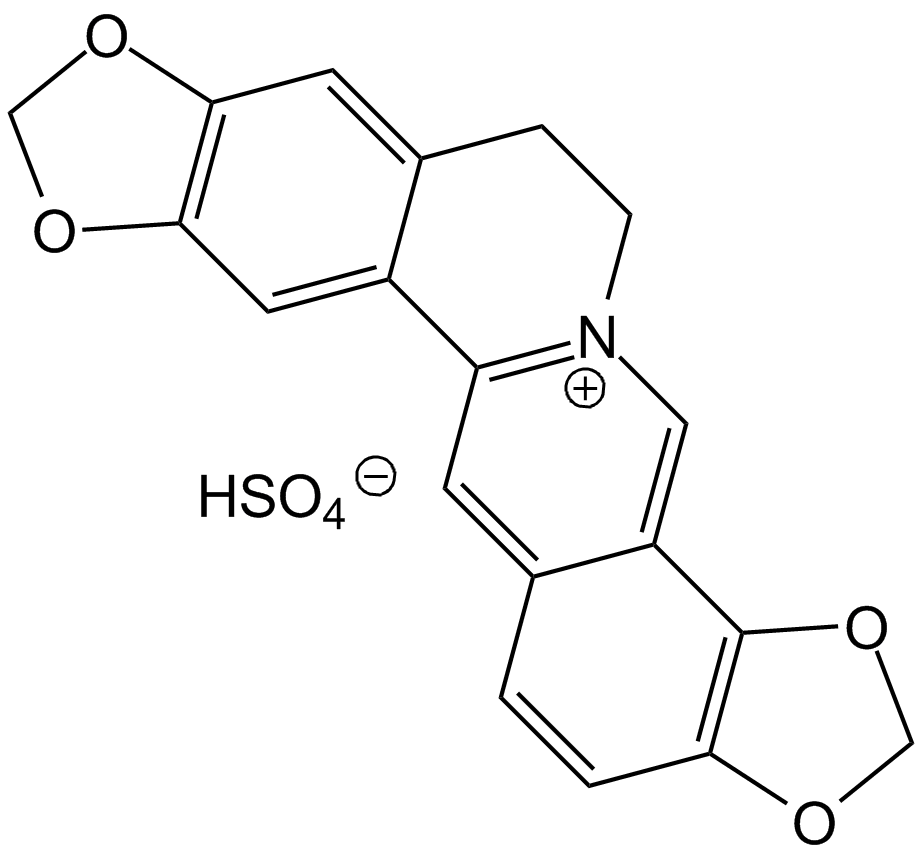
GN10609
Coptisine Sulfate
-
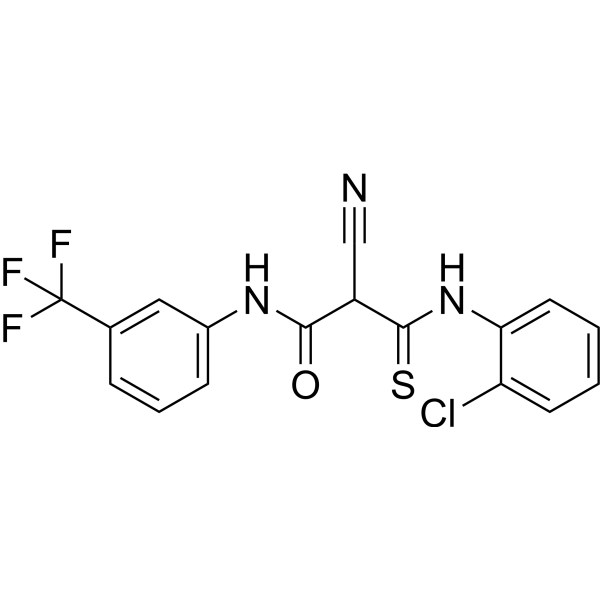
GC67714
DP00477
-
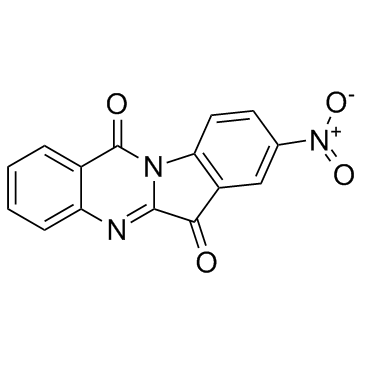
GC33671
GNF-PF-3777 (8-Nitrotryptanthrin)
GNF-PF-3777 (8-Nitrotryptanthrin) (8-Nitrotryptanthrin) is a potent human indoleamine 2,3-dioxygenase 2 (hIDO2) inhibitor which significantly reduces IDO2 activity with Ki of 0.97 μM.
-
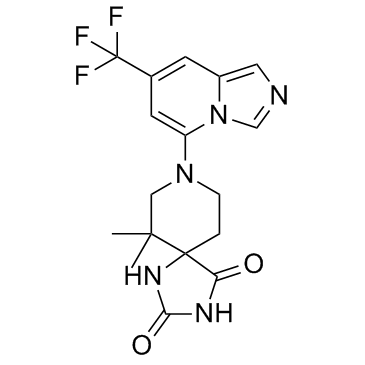
GC34621
IACS-8968
IACS-8968 (IDO/TDO Inhibitor) is a dual IDO and TDO inhibitor, with pIC50s of 6.43 for IDO and <5 for TDO, respectively.
-
GC34622
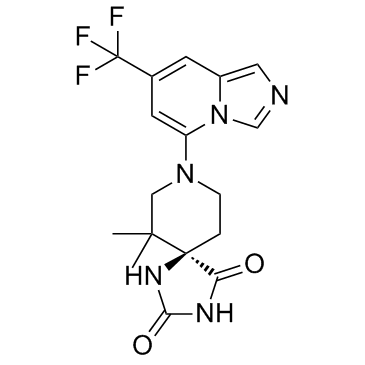
IACS-8968 R-enantiomer
IACS-8968 (R-enantiomer) is the R-enantiomer of IACS-8968. IACS-8968 is a dual IDO and TDO inhibitor, with pIC50s of 6.43 for IDO and <5 for TDO, respectively.
-
GC34623
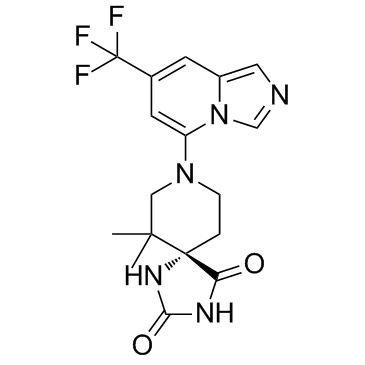
IACS-8968 S-enantiomer
IACS-8968 (S-enantiomer) is the S-enantiomer of IACS-8968. IACS-8968 is a dual IDO and TDO inhibitor, with pIC50s of 6.43 for IDO and <5 for TDO, respectively.
-
GC14425
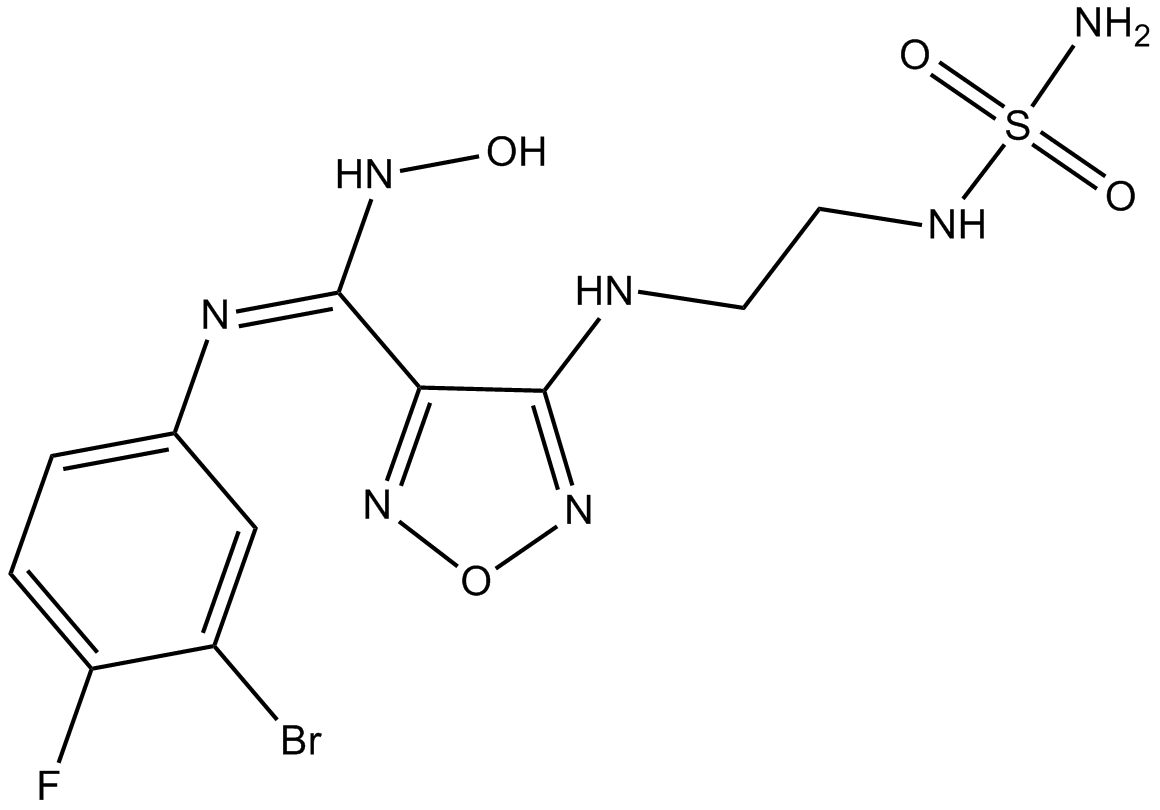
IDO inhibitor 1
Indoleamine-2,3-dioxygenase inhibitor
-
GC15876
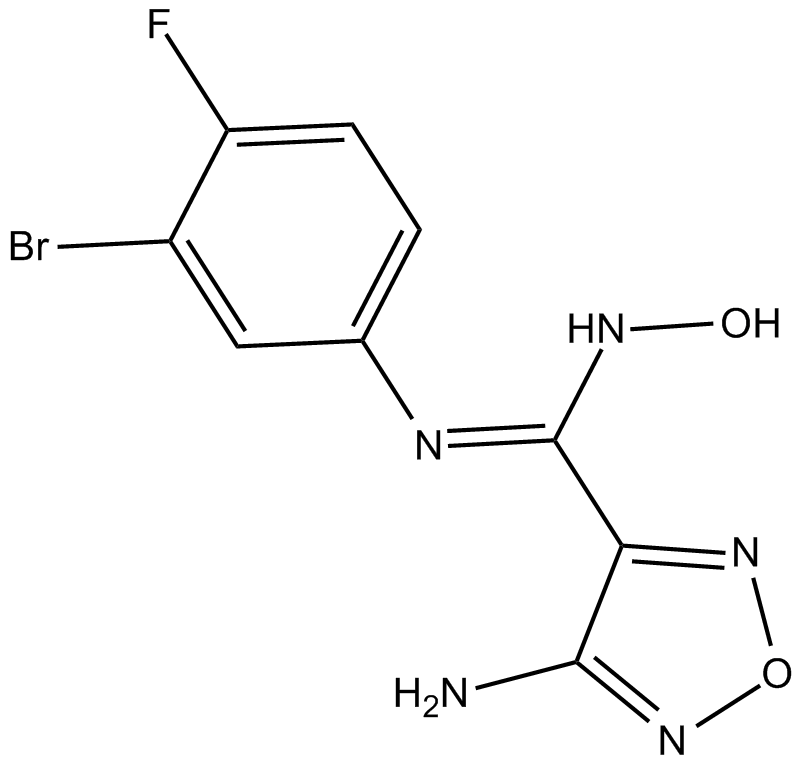
IDO-IN-1
indoleamine-2,3-dioxygenase (IDO) inhibitor
-
GC36295
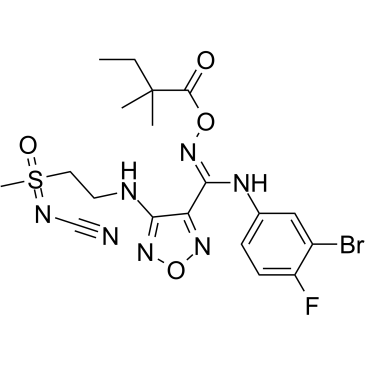
IDO-IN-11
IDO-IN-11 is an indoleamine-2,3-dioxygenase (IDO) inhibitor with IC50s of 0.18 μM (Kinase) and 0.014 μM (Hela Cell), extracted from patent WO 2016041489 A1, compound 13.
-
GC33963
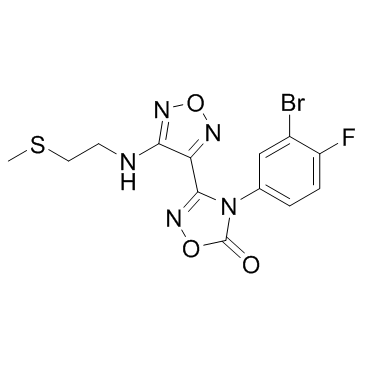
IDO-IN-12
IDO-IN-12 is an indoleamine 2,3-dioxygenase (IDO) inhibitor extracted from patent WO 2017181849 A1.
-
GC33012
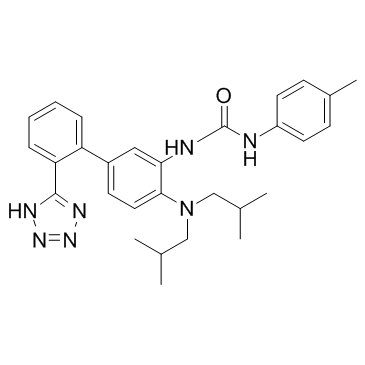
IDO-IN-2
IDO-IN-2 is a potent IDO inhibitor with an IC50 value of 4.52 nM in HeLa cell.
-
GC33058
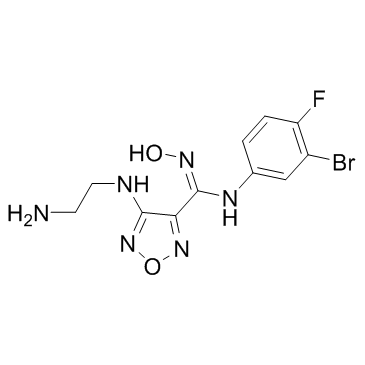
IDO-IN-3
An inhibitor of IDO1
-
GC32899
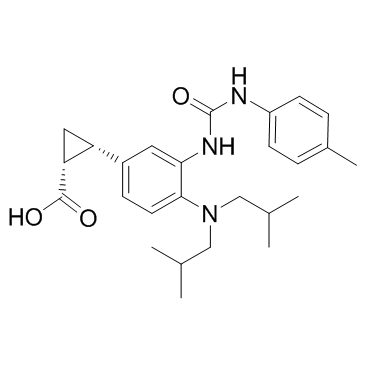
IDO-IN-4
IDO-IN-4 (IDO-IN-4) is an indoleamine 2,3-dioxygenase 1 (IDO-1) inhibitor, extracted from patent WO2014150677A1, Compound example 1 enantiomer 1.
-
GC34137
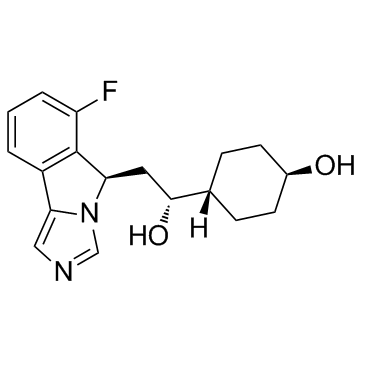
IDO-IN-5 (NLG-1489)
IDO-IN-5 (NLG-1489) (NLG-1489) is an indoleamine 2,3-dioxygenase (IDO) inhibitor extracted from patent WO WO2012142237A1, compound 1489, has an IC50 of 1-10 μM.
-
GC34149
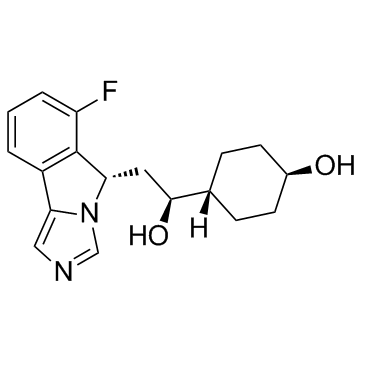
IDO-IN-6 (NLG-1486)
IDO-IN-6 (NLG-1486) (NLG-1486) is an indoleamine 2,3-dioxygenase (IDO) inhibitor extracted from patent WO WO2012142237A1, Compound 1486, has an IC50 of <1 μM.
-
GC34139
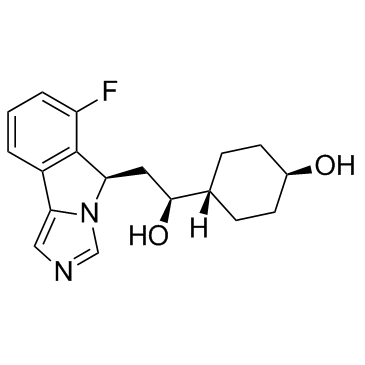
IDO-IN-8 (NLG-1487)
IDO-IN-8 (NLG-1487) (NLG-1487) is an indoleamine 2,3-dioxygenase (IDO) inhibitor extracted from patent WO WO2012142237A1, compound 1487, has an IC50 of 1-10 μM.
-
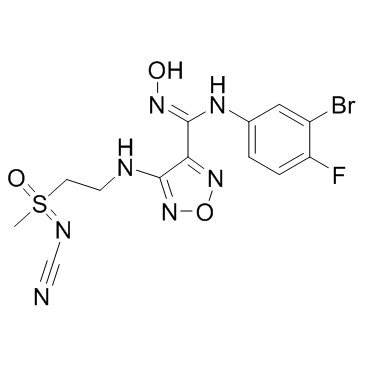
GC33191
IDO-IN-9
IDO-IN-9 is an indoleamine-2,3-dioxygenase (IDO) inhibitor with IC50s of 0.011 μM (Kinase) and 0.0018 μM (Hela Cell), extracted from patent WO 2016041489 A1, compound 6.
-
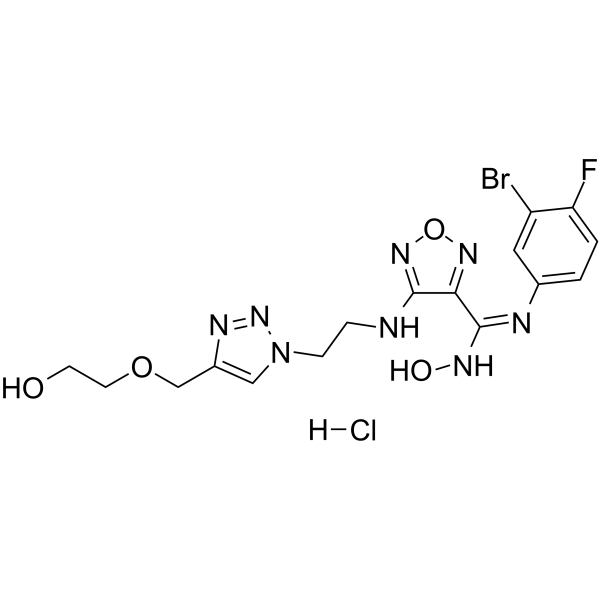
GC36294
IDO/TDO-IN-1
IDO/TDO-IN-1 (compound 25) is a highly potent and orally active dual indoleamine-2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO) inhibitor with IC50s of 9.7 and 47 nM, respectively .
-
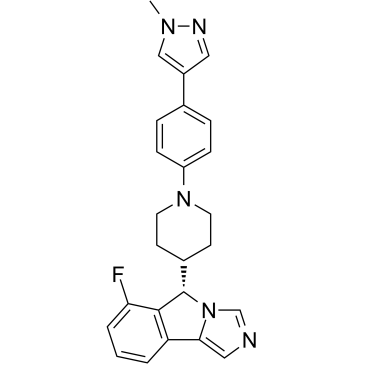
GC65895
IDO1-IN-19
IDO1-IN-19 (Compound 17) is a potent inhibitor of IDO1. IDO1-IN-19 has the potential for the research of cancer diseases.
-
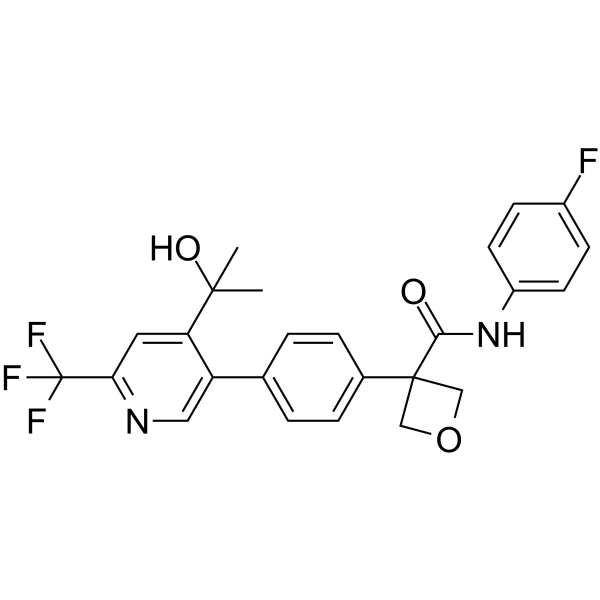
GC65499
IDO1/2-IN-1 hydrochloride
IDO1/2-IN-1 hydrochloride (compound 4t) is the first potent IDO1/IDO2 dual inhibitor with IC50s of 28 nM and 144 nM for IDO1 and IDO2, respectively. IDO1/2-IN-1 hydrochloride exhibits antitumor activies. Orally active.
-
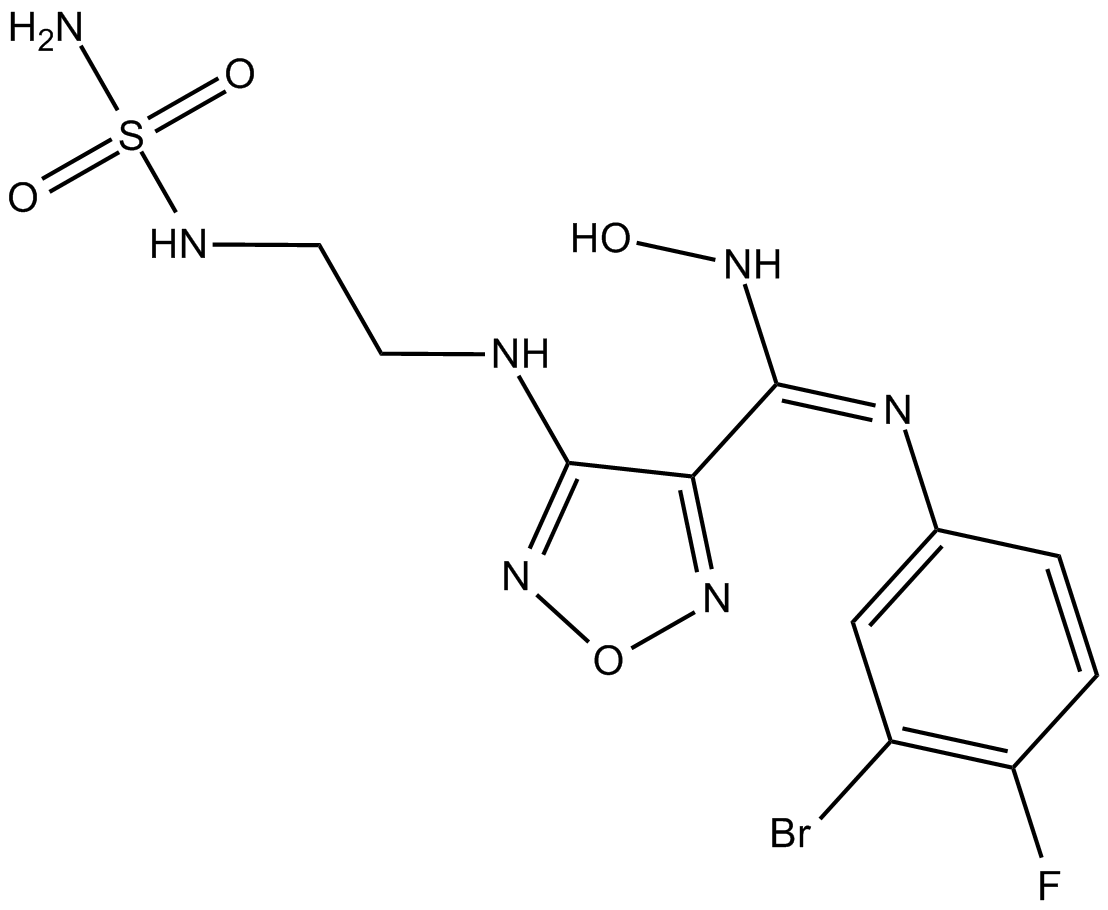
GC17968
INCB-024360
INCB-024360 (INCB 024360) is a potent and selective indoleamine 2,3-dioxigenase 1 (IDO1) inhibitor with an IC50 of 71.8 nM.
-
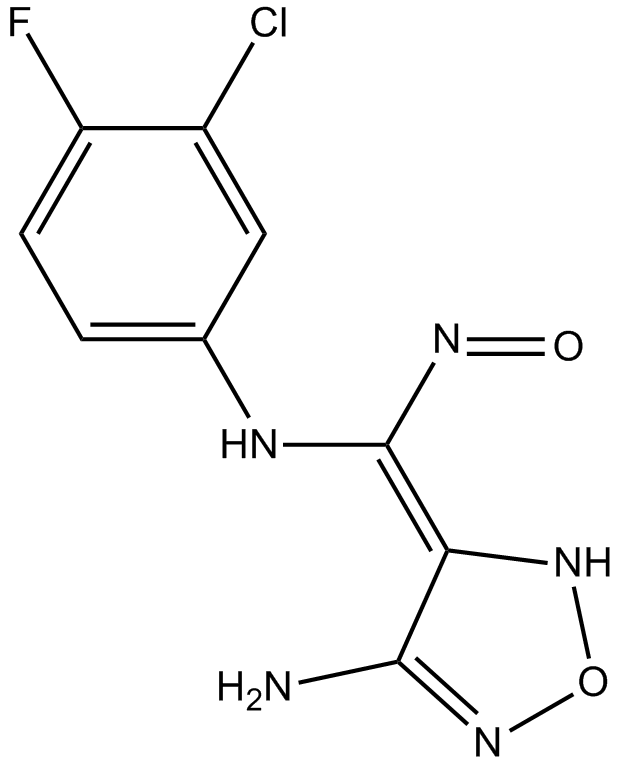
GC15846
INCB024360 analogue
A selective IDO1 inhibitor
-
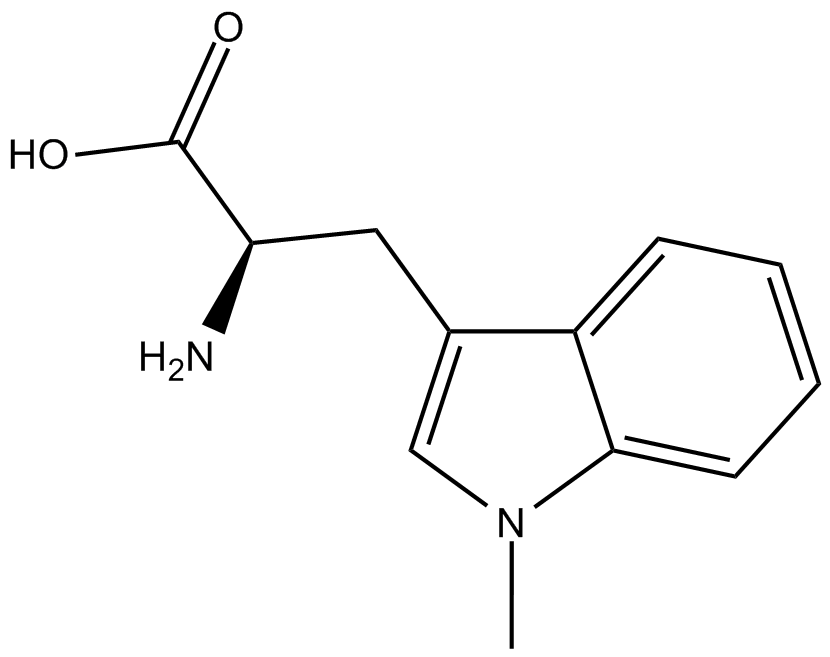
GC11066
Indoximod (NLG-8189)
Indoximod (NLG-8189) (1-Methyl-D-tryptophan) is an orally active indoleamine 2,3-dioxygenase (IDO) pathway inhibitor. Indoximod (NLG-8189) acts as a Trp mimetic in regulating mTOR. Indoximod (NLG-8189) is an immunometabolic adjuvant used for the research of cancer.
-
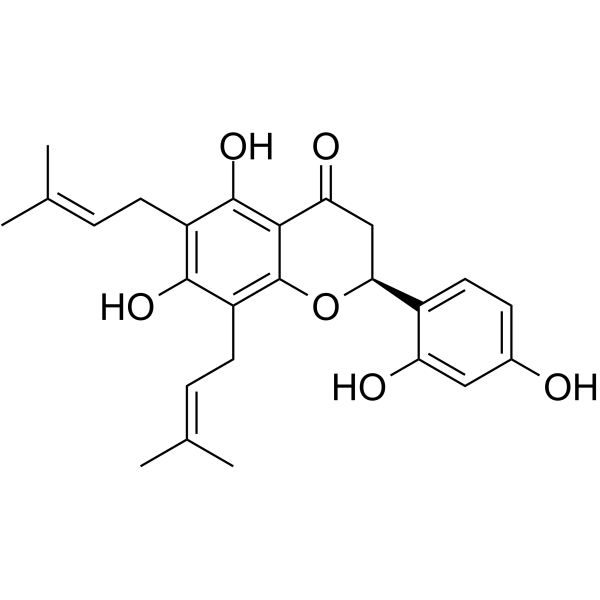
GC64060
Kushenol E
Kushenol E is a class of flavonoids isolated from Sophora flavescens and is a non-competitive indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor with an IC50 of 7.7??M and a Ki of 9.5??M, has anti-tumor activity.
-
GC33287
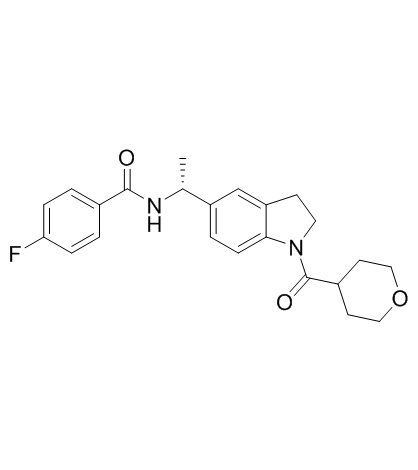
LY-3381916
LY-3381916 is a potent, selective and brain penetrated inhibitor of IDO1 activity, binds to apo-IDO1 lacking heme rather than mature heme-bound IDO1.
-
GC13519
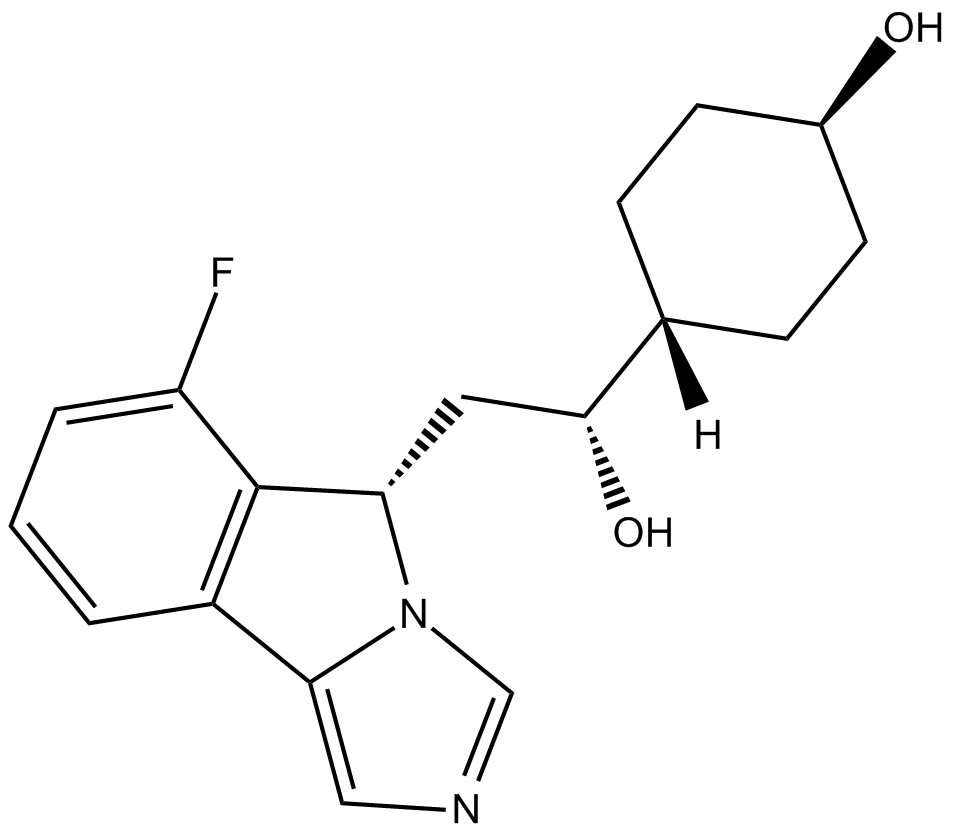
Navoximod
Navoximod (GDC-0919; NLG-?919) is a potent IDO (indoleamine-(2,3)-dioxygenase) pathway inhibitor with Ki/EC50 of 7 nM/75 nM.
-
GC11008
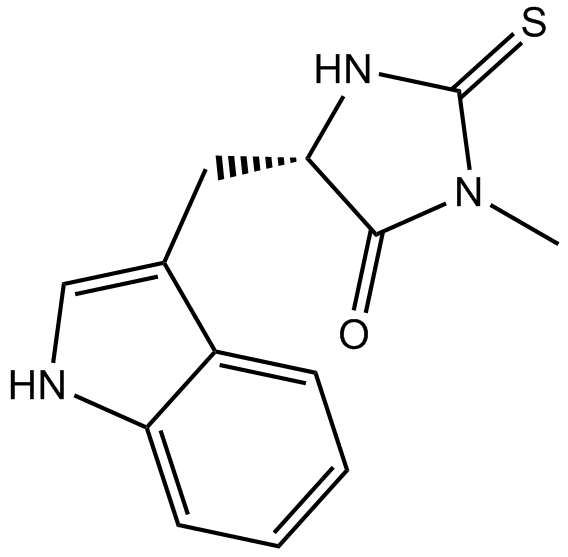
Necrostatin-1
A RIP1 kinase inhibitor
-
GC64955
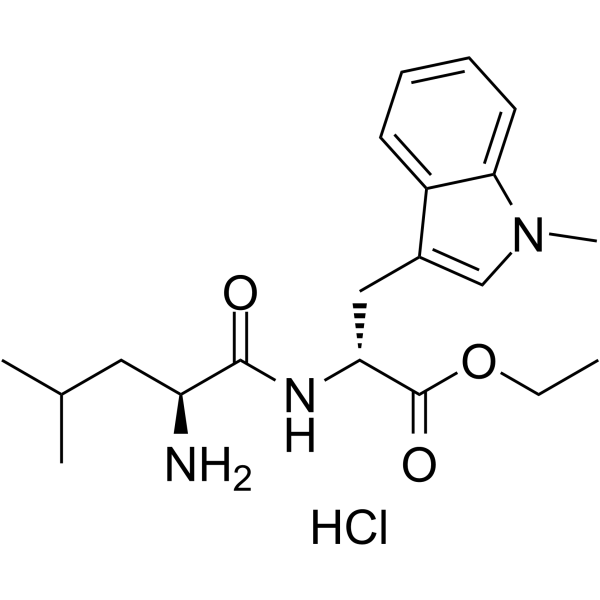
NLG802
NLG802 is a prodrug of indoximod, an orally active indoleamine 2,3-dioxygenase (IDO) inhibitor.
-
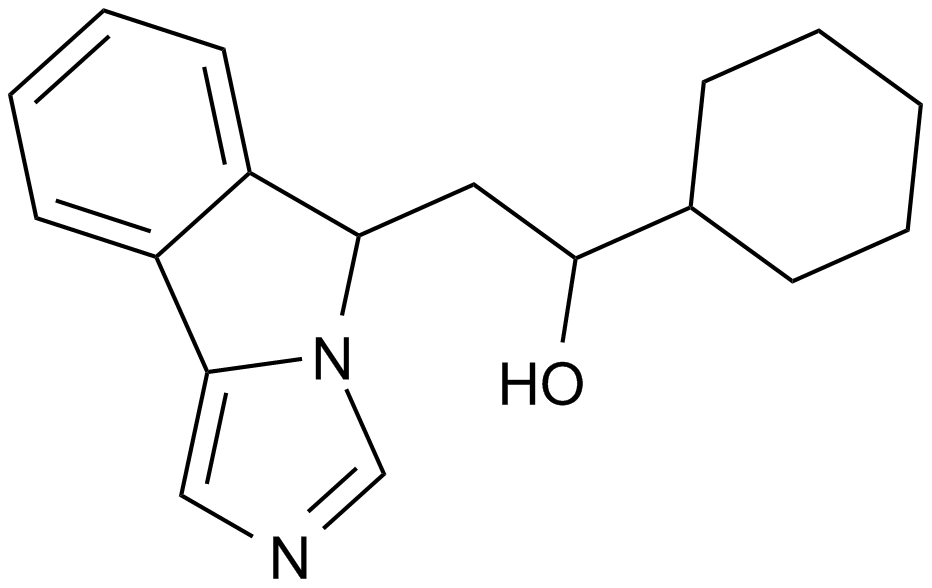
GC13912
NLG919
An IDO pathway inhibitor
-
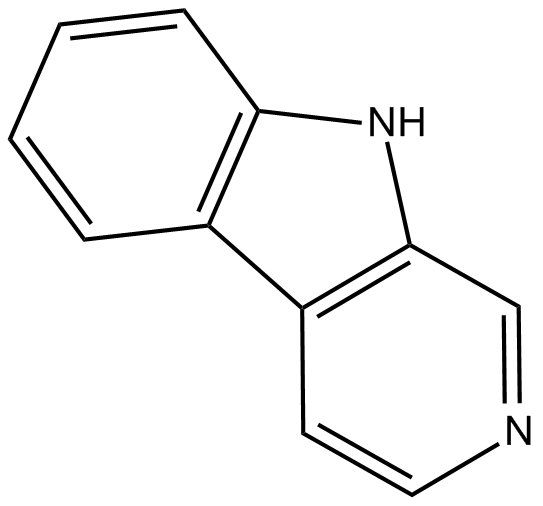
GC15833
Norharmane
inhibitor of indoleamine 2,3-dioxygenase
-
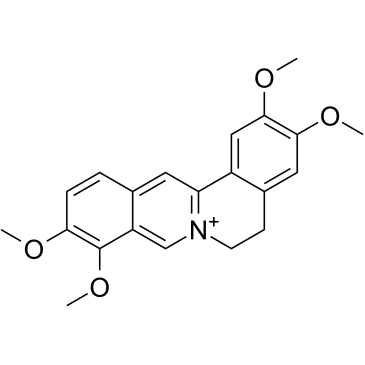
GC39127
Palmatine
Palmatine is an orally active and irreversible indoleamine 2,3-dioxygenase 1 (IDO-1) inhibitor with IC50s of 3 μM and 157μM against HEK 293-hIDO-1 and rhIDO-1, respectively.
-
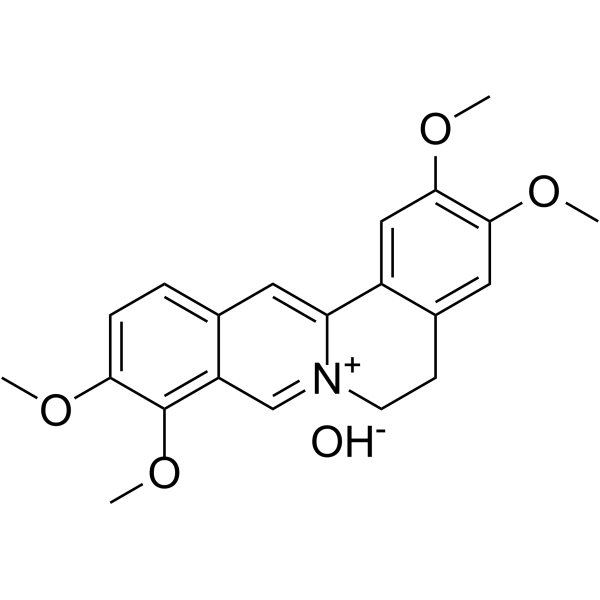
GC68133
Palmatine hydroxide
-
GC32879
PF-06840003 (EOS200271)
PF-06840003 (EOS200271) (EOS200271) is a highly selective orally bioavailable IDO-1 inhibitor with IC50s of 0.41 μM, 0.59 μM, and 1.5 μM for hIDO-1, dIDO-1, and mIDO-1, respectively.
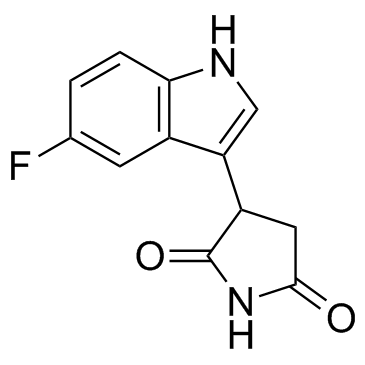
-
GC62689
PROTAC IDO1 Degrader-1
PROTAC IDO1 Degrader-1 is the first potent IDO1 (indoleamine 2,3-dioxygenase 1) degrader that hijacks IDO1 to Cereblon E3 ligase to introduce IDO1 into UPS and eventually achieve ubiquitination and degradation (DC50=2.84 μM).
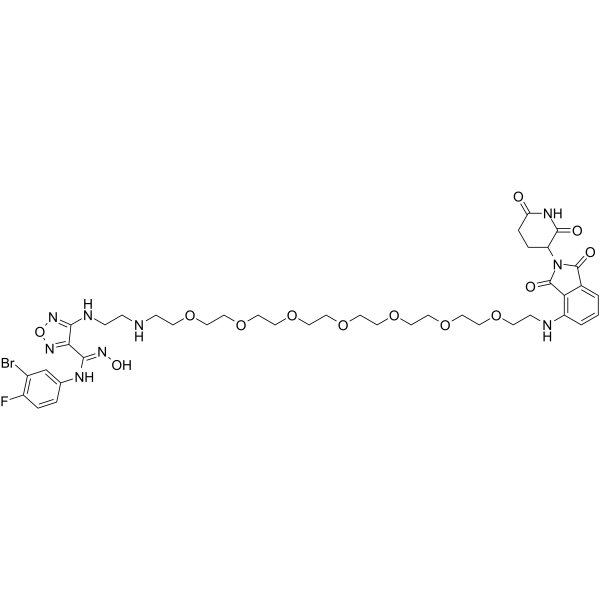
-
GC70167
ZC0109
ZC0109 is a dual inhibitor of IDO1 and thioredoxin reductase 1 (TrxR1), with IC50 values of 50 nM and 3.0 μM, respectively. ZC0109 induces the accumulation of reactive oxygen species (ROS), causing cell cycle arrest at the G1/S phase and leading to apoptosis in cancer cells.