Lipoxygenase
Lipoxygenases (LOXs) are dioxygenases that catalyze the formation of corresponding hydroperoxides from polyunsaturated fatty acids such as linoleic acid and arachidonic acid. Thre are six LOX isoforms have been found in the humans and mice. 5-Lipoxygenase (5-LOX) is a distinct isoform playing an important role in asthma and inflammation. 5-LOX causes the constriction of bronchioles in response to cysteinyl leukotrienes such as LTC4, thus leading to asthma. 5-LOX also induces neutrophilic inflammation by its recruitment in response to LTB4. 12-Lipoxygenase (12-LOX) is an isoform expressed in epithelial cells and myeloid cells including platelets. 12-LOX can be found in the epithelial cells of the skin. 12-LOX is a potential target for novel anti-platelet therapeutics.15-Lipoxygenase (15-LOX) is expressed in epithelial cells and leukocytes, has different substrate specificity in the humans and mice.15-LOX-1 is a target of nonsteroidal anti-inflammatory drug-induced apoptosis in colorectal cancer cells.
Targets for Lipoxygenase
Products for Lipoxygenase
- Cat.No. Product Name Information
-
GC38000
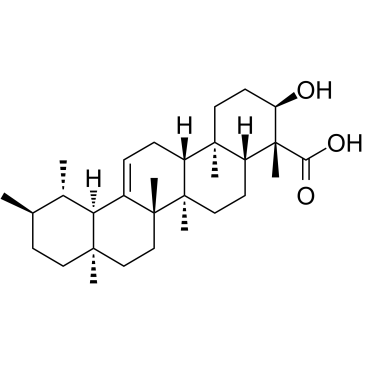
β-Boswellic acid
A pentacyclic triterpene with diverse bioactivities
-
GC41649
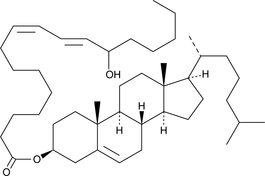
(±)13-HODE cholesteryl ester
(±)13-HODE cholesteryl ester was originally extracted from atherosclerotic lesions and shown to be produced by Cu2+-catalyzed oxidation of LDL.
-
GC40828
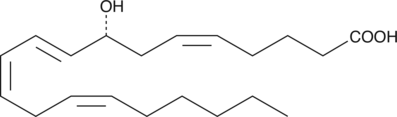
(±)5-HETE lactone
(±)5-HETE lactone is a cyclic ester formed by acid-catalyzed nucleophilic addition of the C-5 hydroxyl to the C-1 carboxyl of (±)5-HETE.
-
GC41666
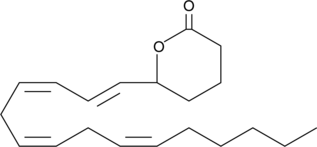
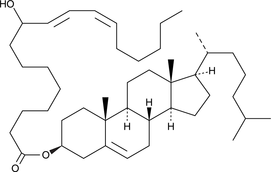
(±)9-HODE cholesteryl ester
(±)9-HODE cholesteryl ester was originally extracted from atherosclerotic lesions and shown to be produced by Cu2+-catalyzed oxidation of LDL.
-
GC41770
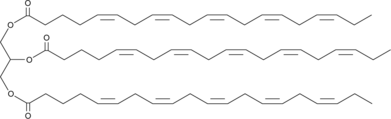
1,2,3-Trieicosapentaenoyl-rac-glycerol
1,2,3-Trieicosapentaenoyl-rac-glycerol (EPA-TG) is a glycerol ester of eicosapentaenoic acid, which is an ω-3 fatty acid.
-
GC45948
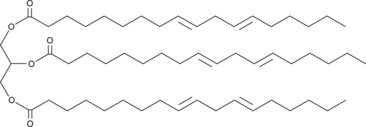
1,2,3-Trilinoelaidoyl-rac-glycerol
A triacylglycerol
-
GC42039
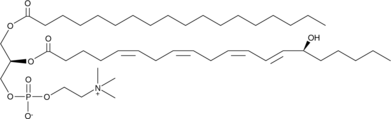
1-Stearoyl-2-15(S)-HETE-sn-glycero-3-PC
1-Stearoyl-2-15(S)-HETE-sn-glycero-3-PC is a phospholipid that contains stearic acid at the sn-1 position and 15(S)-HETE at the sn-2 position.
-
GC42040
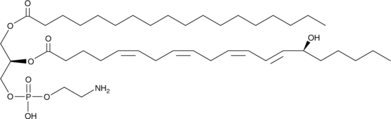
1-Stearoyl-2-15(S)-HETE-sn-glycero-3-PE
1-Stearoyl-2-15(S)-HETE-sn-glycero-3-PE is a phospholipid that contains stearic acid at the sn-1 position and 15(S)-HETE at the sn-2 position.
-
GC49730
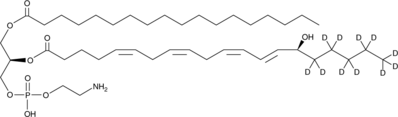
1-Stearoyl-2-15(S)-HETE-sn-glycero-3-PE-d11
An internal standard for the quantification of 1-stearoyl-2-15(S)-HETE-sn-glycero-3-PE
-
GC42041
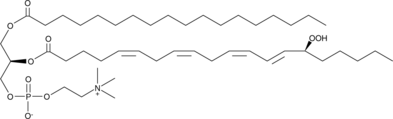
1-Stearoyl-2-15(S)-HpETE-sn-glycero-3-PC
1-Stearoyl-2-15(S)-HpETE-sn-glycero-3-PC is a phospholipid that contains stearic acid at the sn-1 position and 15(S)-HpETE at the sn-2 position.
-
GC42042
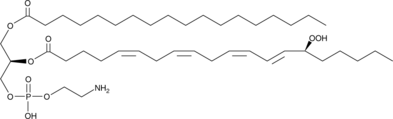
1-Stearoyl-2-15(S)-HpETE-sn-glycero-3-PE
1-Stearoyl-2-15(S)-HpETE-sn-glycero-3-PE is a phospholipid that contains stearic acid at the sn-1 position and 15(S)-HpETE at the sn-2 position.
-
GC40971
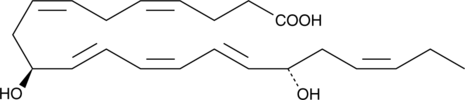
10(S),17(S)-DiHDHA
Protectin D1 (also known as neuroprotectin D1 when produced in neuronal tissues) is a DHA-derived dihydroxy fatty acid that exhibits potent protective and anti-inflammatory activities.
-
GC40368
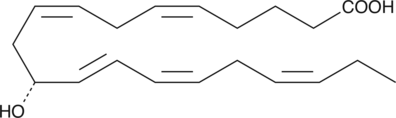
11(R)-HEPE
11(R)-HEPE is produced by the oxidation of EPA by 11(R)-LO.
-
GC40445
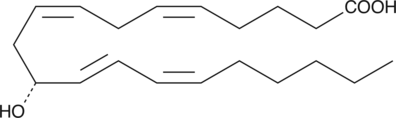
11(R)-HETE
11(R)-HETE is biosynthesized by 11(R)-LOs of the sea urchin, S.
-
GC10821
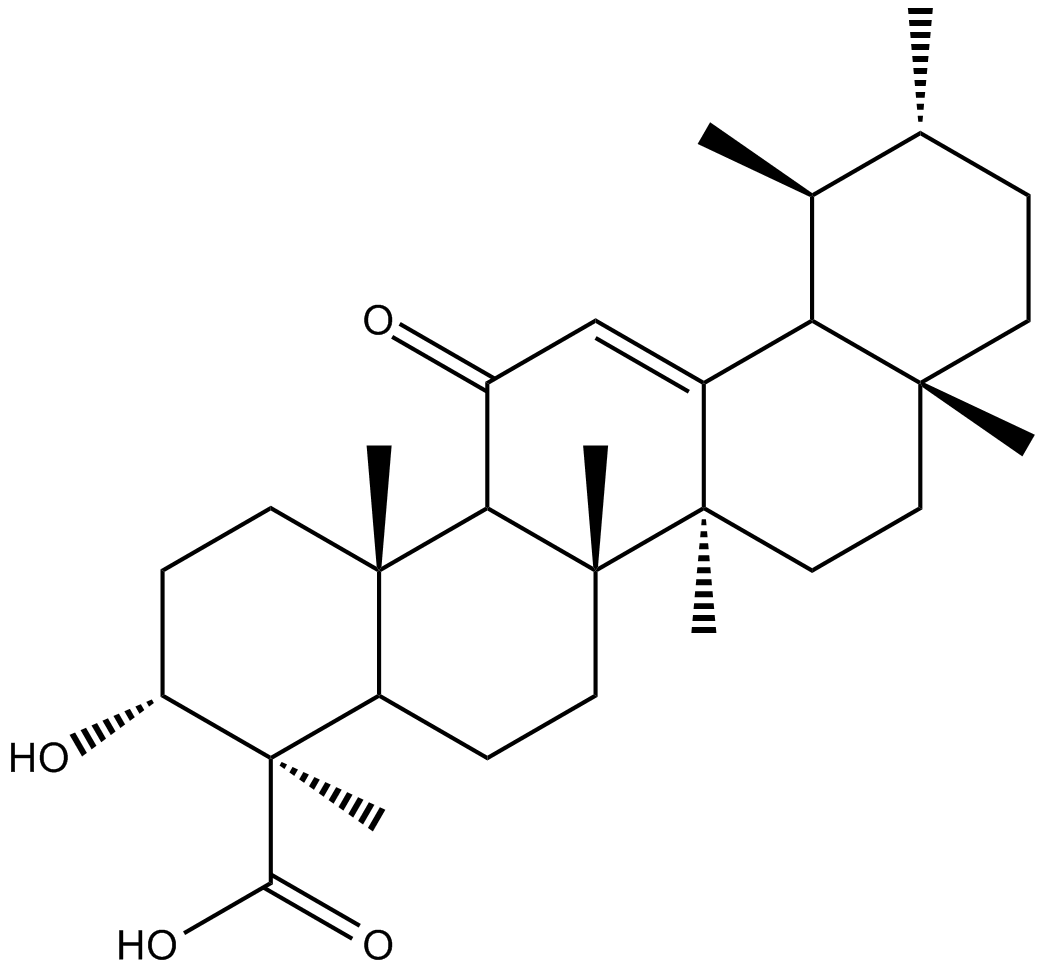
11-keto-β-Boswellic Acid
11-Keto-beta-boswellic acid (11-Keto-β-boswellic acid) is a pentacyclic triterpenic acid of the oleogum resin from the bark of the Boswellia serrate tree, popularly known as Indian Frankincense. 11-Keto-beta-boswellic acid has the anti-inflammatory activity is primarily due to inhibit 5-lipoxygenase (5-LOX) and subsequent leukotriene and nuclear factor-kappa B (NF-κB) activation and tumor necrosis factor alpha generation production.
-
GC41144
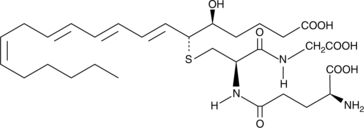
11-trans Leukotriene C4
11-trans Leukotriene C4 (11-trans LTC4) is a C-11 double bond isomer of LTC4.
-
GC41147
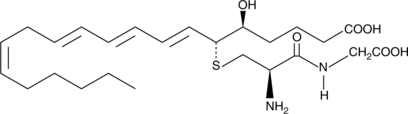
11-trans Leukotriene D4
11-trans Leukotriene D4 (11-trans LTD4) is a C-11 double bond isomer of LTD4.
-
GC41149
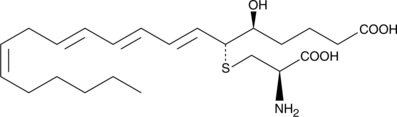
11-trans Leukotriene E4
Slow isomerization of the C-11 double bond of LTE4 leads to the formation of 11-trans LTE4.
-
GC40447
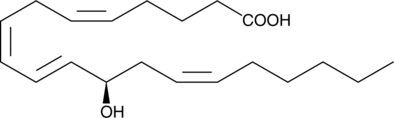
12(R)-HETE
Biosynthesis of 12(R)-HETE in invertebrates is via lipoxygenation of arachidonic acid.
-
GC40371
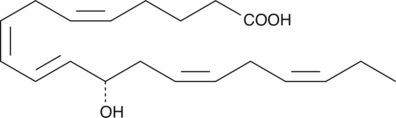
12(S)-HEPE
12(S)-HEPE is a monohydroxy fatty acid synthesized from EPA by the action of 12-LO.
-
GC40448
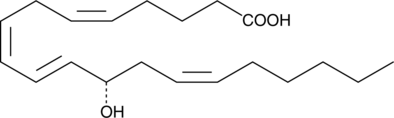
12(S)-HETE
12(S)-HETE is the predominant lipoxygenase product of mammalian platelets.
-
GC41882
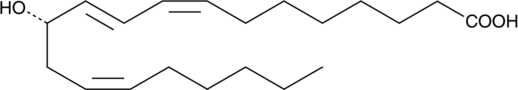
12(S)-HETrE
12(S)-HETrE is produced by 12-lipoxygenase oxidation of dihomo-γ-linolenic acid (DGLA).
-
GC41095
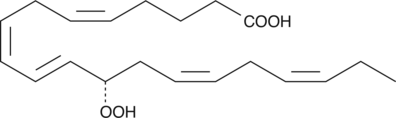
12(S)-HpEPE
12(S)-HpEPE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 12-lipoxygenase on eicosapentaenoic acid.
-
GC41122
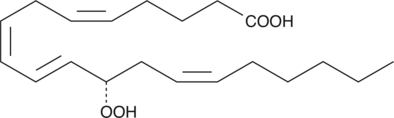
12(S)-HpETE
12(S)-HpETE is a monohydroperoxy polyunsaturated fatty acid (PUFA) produced by the action of platelet or leukocyte 12-lipoxygenase (12-LO) on arachidonic acid.
-
GC41123
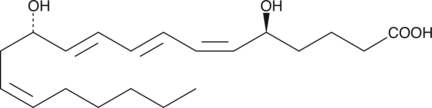
12-epi Leukotriene B4
Leukotriene B4 (LTB4) compounds are produced by both enzymatic and non-enzymatic processes.
-
GC41096
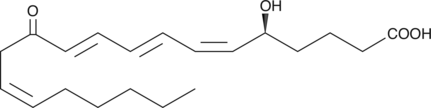
12-oxo Leukotriene B4
Leukotriene B4 (LTB4) is a dihydroxy fatty acid derived from arachidonic acid through the 5-LO pathway.
-
GC46418
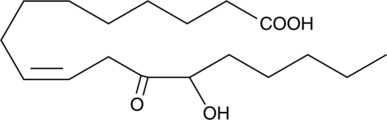
12-oxo-13-HOME
An oxylipin
-
GC40372
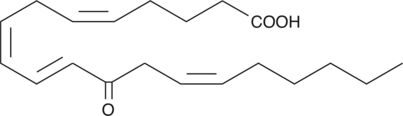
12-OxoETE
12-OxoETE is synthesized by human platelets and Aplysia nervous tissue after incubation with arachidonic acid.
-
GC19462
13(R)-HODE
13(R)-HODE is the opposite enantiomer of the 13(S)-HODE produced when linoleic acid is incubated with soybean lipoxygenase.

-
GC19463
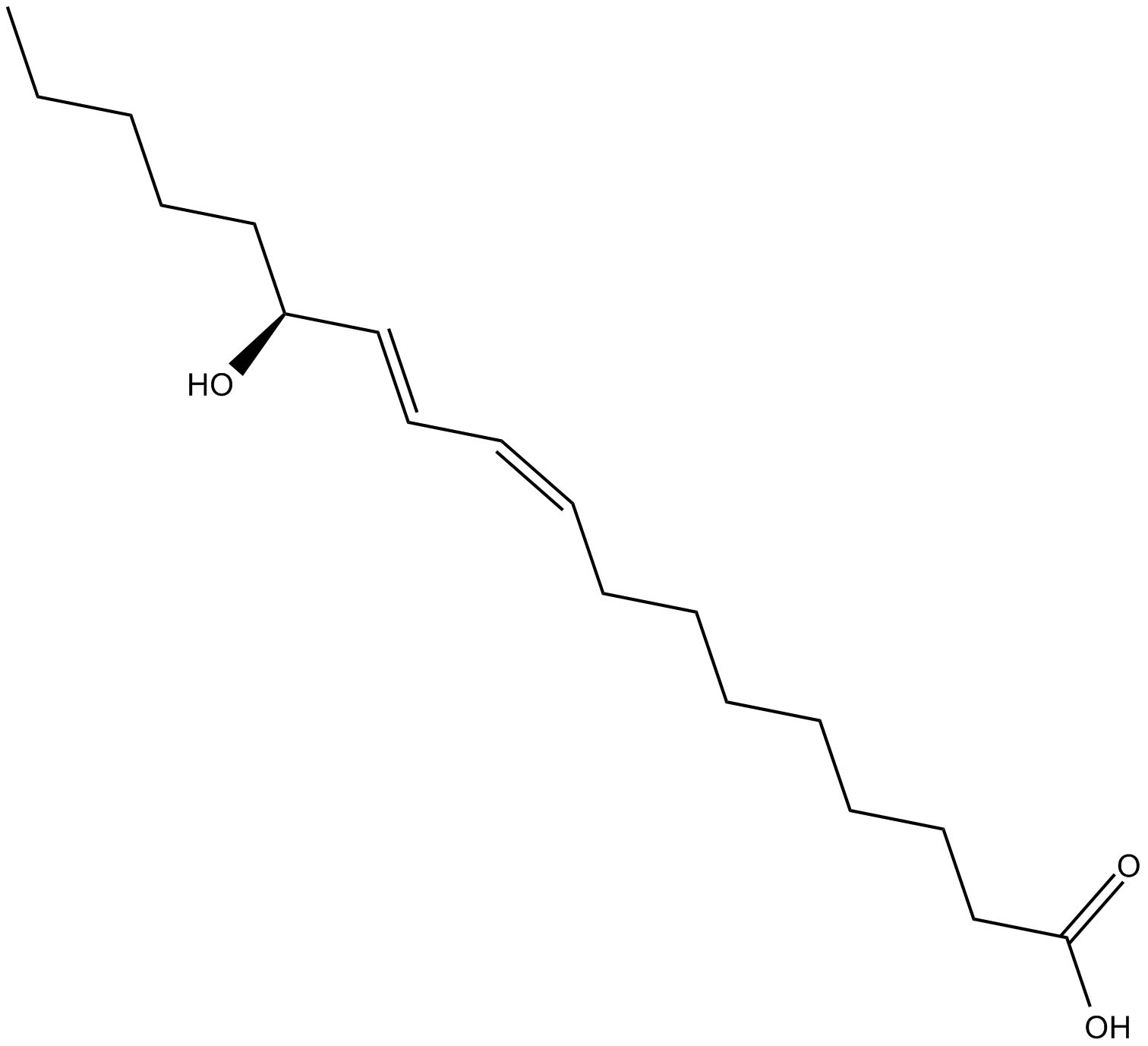
13(S)-HODE
13(S)-HODE (13(S)-HODE), the product of 15-lipoxygenase (15-LOX) metabolism of linoleic acid, functions as the endogenous ligand to activate PPARγ.
-
GC41220
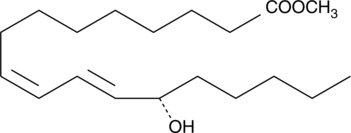
13(S)-HODE methyl ester
13(S)-hydroxyoctadecadienoic acid (13(S)-HODE) is a 15-lipoxygenase metabolite of linoleic acid produced in endothelial cells, leukocytes, and tumor cells.
-
GC41896
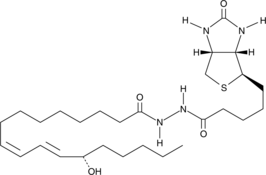
13(S)-HODE-biotin
13(S)-HODE is the lipoxygenase metabolite of linoleic acid.
-
GC46420
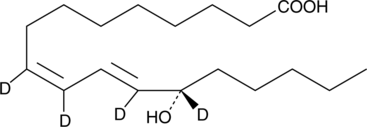
13(S)-HODE-d4
An internal standard for the quantification of 13-HODE
-
GC41897
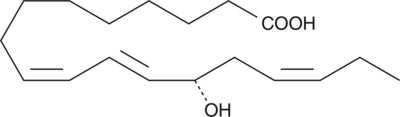
13(S)-HOTrE
13(S)-HOTrE is the 15-lipoxygenase (15-LO) product of linolenic acid.
-
GC41898
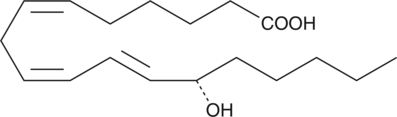
13(S)-HOTrE(γ)
13(S)-HOTrE(γ) is the 15-LO product of γ-linolenic acid.
-
GC19474
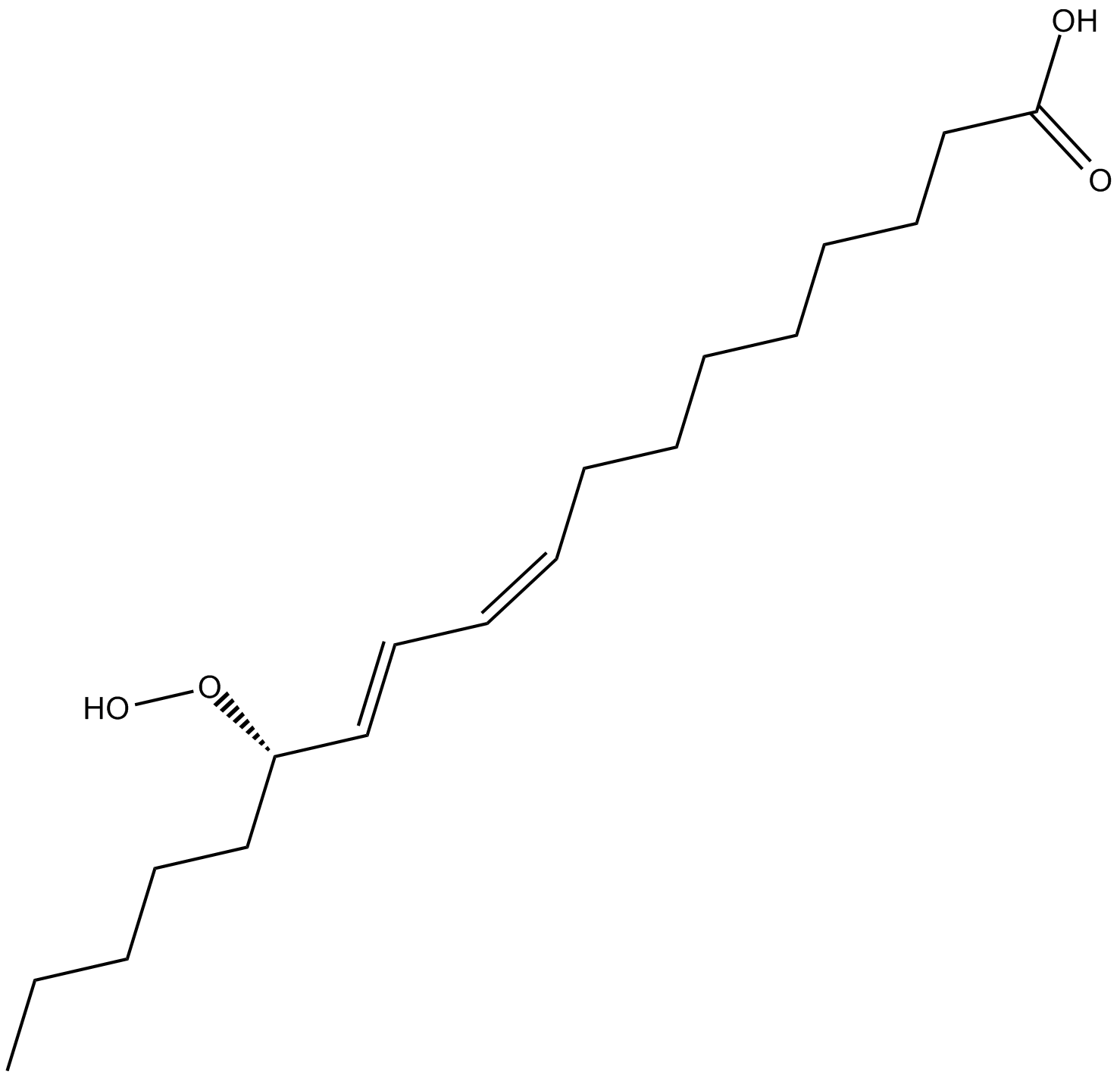
13(S)-HpODE
13(S)-HpODE is produced by the oxidation of linoleic acid by lipoxygenase-1 (LO-1) in many plants including soybean, flaxseed, apples, and tea leaves,1,2 and by 15-LO in mammals.
-
GC41899
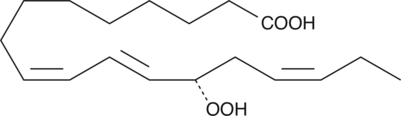
13(S)-HpOTrE
13(S)-HpOTrE is a monohydroperoxy polyunsaturated fatty acid produced in soybeans by the action of soybean LO-2 on esterified α-linolenic acid.
-
GC41900
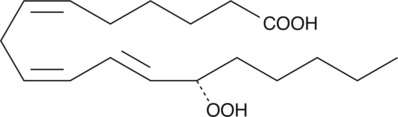
13(S)-HpOTrE(γ)
13(S)-HpOTrE(γ) is a monohydroxy PUFA produced by the action of soybean lipoxygenase-1 (LO-1) on γ-linolenic acid.
-
GC41911
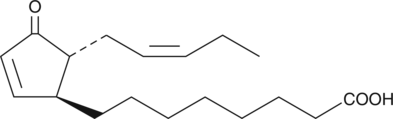
13-epi-12-oxo Phytodienoic Acid
13-epi-12-oxo Phytodienoic acid (13-epi-12-oxo PDA) is a lipoxygenase metabolite of α-linolenic acid in the leaves of green plants such as corn.
-
GC41206
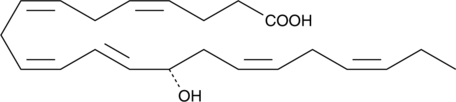
14(S)-HDHA
Docosahexaenoic acid is a nutritionally-derived ω-3 fatty acid that is abundant in the brain and the retina and is thought to be important in early development and for therapeutic approaches to inflammatory disorders and cancer.
-
GC41100
14,15-dehydro Leukotriene B4
Leukotriene B4 (LTB4) is a dihydroxy fatty acid derived from arachidonic acid through the 5-lipoxygenase pathway.
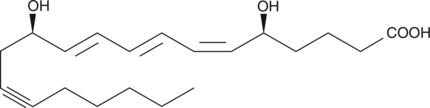
-
GC41145
14,15-Leukotriene C4
Leukotrienes (LTs) are a group of acute inflammatory mediators derived from arachidonic acid in leukocytes.
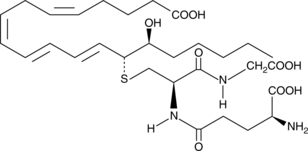
-
GC41148
14,15-Leukotriene D4
14,15-Leukotriene D4 (14,15-LTD4) is a member of an alternate class of LTs synthesized by a pathway involving the dual actions of 15- and 12-lipoxygenases (15- and 12-LOs) on arachidonic acid via 15-HpETE and 14,15-LTA4 intermediates.
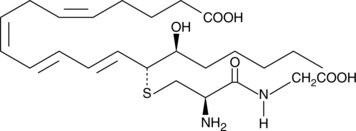
-
GC41150
14,15-Leukotriene E4
Leukotrienes (LTs) are a group of acute inflammatory mediators derived from arachidonic acid in leukocytes.
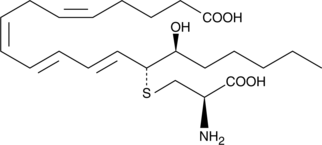
-
GC41415
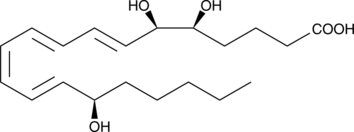
15(R)-Lipoxin A4
Lipid-derived lipoxins are produced at the site of vascular and mucosal inflammation where they down-regulate polymorphonuclear leukocyte recruitment and function.
-
GC40427
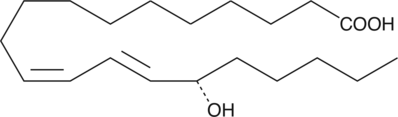
15(S)-HEDE
15(S)-HEDE is produced from 11Z,14Z-eicosadienoic acid by 15-LO.
-
GC40373
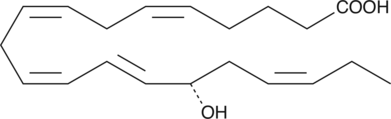
15(S)-HEPE
15(S)-HEPE is a monohydroxy fatty acid synthesized from EPA by the action of 15-LO.
-
GC40451
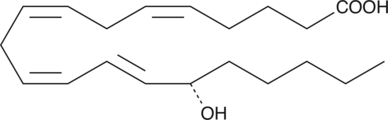
15(S)-HETE
15(S)-HETE is a major arachidonic acid metabolite from the 15-lipoxygenase pathway.
-
GC41925
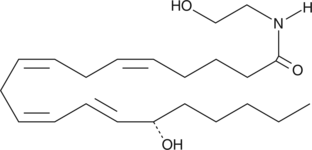
15(S)-HETE Ethanolamide
Arachidonoyl ethanolamide was the first endogenous cannabinoid (CB) to be isolated and characterized as an agonist acting on the same receptors (CB1 and CB2) as THC.
-
GC40839
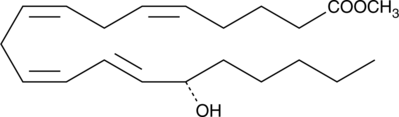
15(S)-HETE methyl ester
15(S)-HETE methyl ester is a synthetic derivative of 15(S)-HETE, a major arachidonic acid metabolite from the 15-lipoxygenase pathway.
-
GC46442
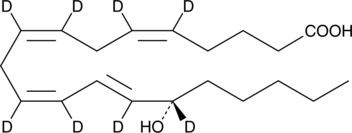
15(S)-HETE-d8
An internal standard for the quantification of 15-HETE
-
GC49894
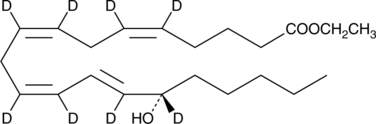
15(S)-HETE-d8 ethyl ester
An internal standard for the quantification of 15(S)-HETE ethyl ester
-
GC41927
15(S)-HETrE
15(S)-HETrE is the hydroxy-trienoic acid resulting from 15-lipoxygenation of dihomo-γ-linolenic acid.
-
GC41403
15(S)-HpEDE
15(S)-HpEDE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 15-lipoxygenase on eicosadienoic acid.
-
GC41101
15(S)-HpEPE
15(S)-HpEPE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 15-lipoxygenase on eicosapentaenoic acid.
-
GC41124
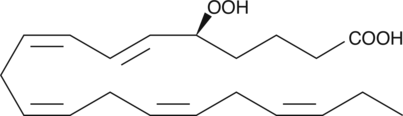
15(S)-HpETE
15(S)-HpETE is a monohydroperoxy polyunsaturated fatty acid (PUFA) produced by the action of 15-lipoxygenase (15-LO) on arachidonic acid.
-
GC41938
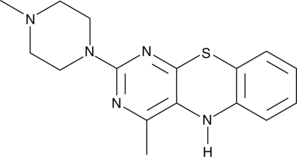
15-Lipoxygenase Inhibitor 1
15-Lipoxygenase Inhibitor 1 is a selective inhibitor of 15-lipoxygenase, with an IC50 of 18 μM. 15-Lipoxygenase Inhibitor 1 has IC50s of 19.5 μM and 19.1 μM for soybean 15-lipoxygenase (SLO) and human 15-lipoxygenase-1 (15-LOX-1), respectively. 15-Lipoxygenase Inhibitor 1 has potential for the research of prostate cancer.
-
GC41940
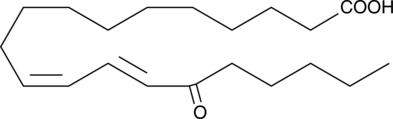
15-OxoEDE
15-OxoEDE is produced by the oxidation of 15-HEDE.
-
GC40376
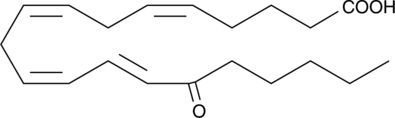
15-OxoETE
15-OxoETE is produced by oxidation of the 15-hydroxyl of 15-HETE.
-
GC41951
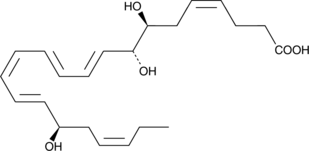
17(R)-Resolvin D1
Resolvins are a family of potent lipid mediators derived from both eicosapentaenoic acid and docosahexaenoic acid.
-
GC41227
17(R)-Resolvin D1 methyl ester
17(R)-Resolvin D1 (17(R)-RvD1) is an aspirin-triggered epimer of RvD1 that reduces human polymorphonuclear leukocyte transendothelial migration, the earliest event in acute inflammation, with equipotency to RvD1 (EC50 = ~30 nM).
-
GC41952
17(R)-Resolvin D4
17(R)-Resolvin D4 (17(R)-RvD4) is an aspirin-triggered epimer of RvD4 .
-
GC41208
17(S)-HDHA
17(S)-HDHA is a primary mono-oxygenation product of docosahexaenoic acid in human whole blood, human leukocytes, and mouse brain.
-
GC49356
17(S)-HDoTE
A metabolite of adrenic acid
-
GC40975
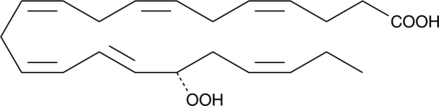
17(S)-HpDHA
17(S)-HpDHA is a mono-oxygenation product of docosahexaenoic acid in human whole blood, human leukocytes, human glial cells, and mouse brain.
-
GC41529
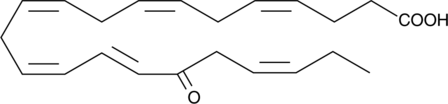
17-oxo-4(Z),7(Z),10(Z),13(Z),15(E),19(Z)-Docosahexaenoic Acid
17-oxo-4(Z),7(Z),10(Z),13(Z),15(E),19(Z)-Docosahexaenoic acid is a metabolite of lipoxygenase-mediated oxidation of DHA that is produced endogenously by aspirin-enhanced COX-2 activity.
-
GC41209
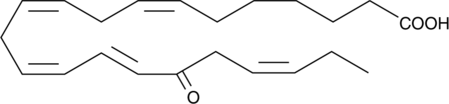
17-oxo-7(Z),10(Z),13(Z),15(E),19(Z)-Docosapentaenoic Acid
Docosapentaenoic acid (DPA) is a ω-3 fatty acid found in fish oils.
-
GC41980
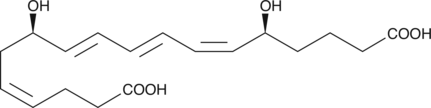
18-carboxy dinor Leukotriene B4
18-carboxy dinor Leukotriene B4 (18-carboxy dinor LTB4) is a β-oxidation metabolite of LTB4.
-
GC46057
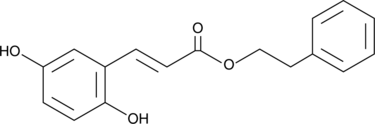
2,5-Dihydroxycinnamic Acid phenethyl ester
An inhibitor of 5-LO
-
GC42082
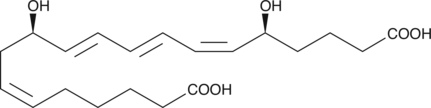
20-carboxy Leukotriene B4
20-carboxy LTB4 is a metabolite of LTB4 in human neutrophils.
-
GC41421
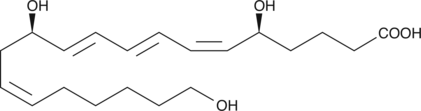
20-hydroxy Leukotriene B4
20-hydroxy LTB4 is a metabolite of LTB4 in human neutrophils.
-
GC49849
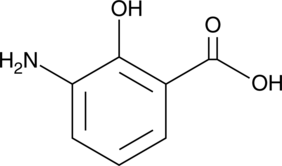
3-Aminosalicylic Acid
A salicylic acid derivative
-
GC49364
3-Hydroxycoumarin
A coumarin with diverse biological activities
-
GC64078
3-O-Acetyl-11-hydroxy-beta-boswellic acid
3-O-Acetyl-11-hydroxy-beta-boswellic acid is a potent 5-lipoxygenase (5-LO) inhibitor.
-
GC30277
4',5-Dihydroxyflavone
4',5-Dihydroxyflavone is a soybean LOX-1 and yeast α-Glucosidase inhibitor, with an Ki of 102.6 μM for soybean LOX-1 and an IC50 of 66 μM for yeast α-glucosidase.
-
GC64621
4-Nitrocatechol
4-Nitrocatechol is a potent lipoxygenase inhibitor.
-
GC40459
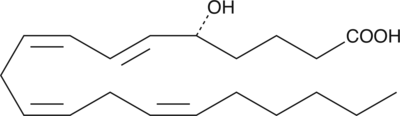
5(R)-HETE
5(R)-HETE is a rare lipoxygenase product of arachidonic acid.
-
GC41126
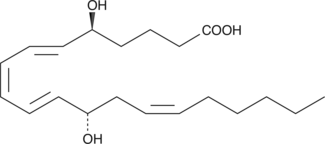
5(S),12(S)-DiHETE
5(S),12(S)-DiHETE is a natural bioactive lipid derived from arachidonic acid.
-
GC41127
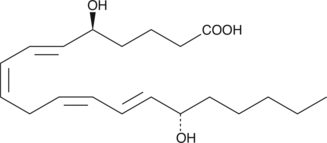
5(S),15(S)-DiHETE
5(S),15(S)-DiHETE is synthesized by 15-LO from 5(S)-HETE.
-
GC41128
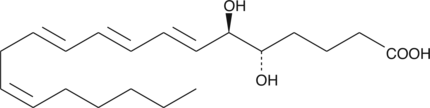
5(S),6(R)-11-trans DiHETE
5(S),6(R)-11-trans DiHETE is a C-11 double bond isomer of 5(S),6(R)-DiHETE that is formed by the enzymatic isomerization of 5(S),6(R)-DiHETE by a membrane bound factor.
-
GC41129
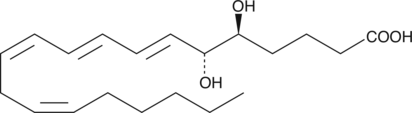
5(S),6(R)-DiHETE
5(S),6(R)-DiHETE is a dihydroxy polyunsaturated fatty acid and a nonenzymatic hydrolysis product of leukotriene A4 (LTA4).
-
GC41131
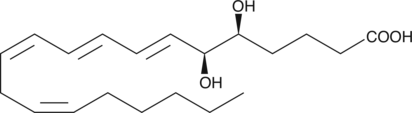
5(S),6(S)-DiHETE
5(S),6(S)-DiHETE is one of the four diastereomeric 5,6-dihydroxy acids produced from the non-enzymatic hydrolysis of LTA4.
-
GC40378
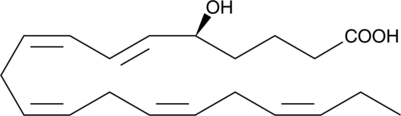
5(S)-HEPE
5(S)-HEPE is produced by 5-lipoxygenase catalyzed oxidation of eicosapentaenoic acid (EPA).
-
GC40460
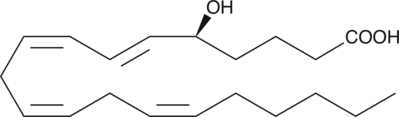
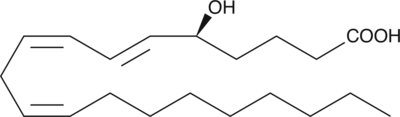
5(S)-HETE
5(S)-HETE is produced by the action of 5-LO on arachidonic acid to give 5(S)-HpETE, followed by reduction of the hydroperoxide.
-
GC40829
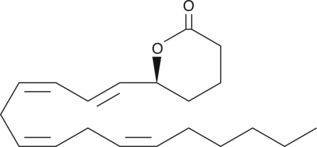
5(S)-HETE lactone
5(S)-HETE lactone is a cyclic ester formed by acid-catalyzed nucleophilic addition of the C-5 hydroxyl to the C-1 carboxyl of 5(S)-HETE.
-
GC46679
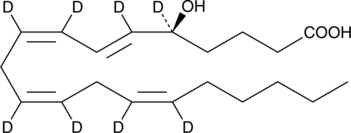
5(S)-HETE-d8
An internal standard for the quantification of 5-HETE
-
GC42479
5(S)-HETrE
5(S)-HETrE is produced by the action of 5-LO when mead acid is the substrate.
-
GC41105
5(S)-HpEPE
5(S)-HpEPE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 5-LO on EPA.
-
GC40784
5(Z),8(Z),14(Z)-Eicosatrienoic Acid
5(Z),8(Z),14(Z)-Eicosatrienoic acid is a polyunsaturated fatty acid that can be a substrate for 5-lipoxygenase (5-LO).
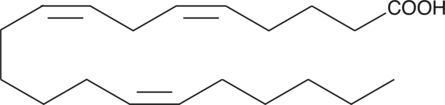
-
GC40830
5,6-dehydro Arachidonic Acid
5,6-dehydro Arachidonic acid is an analog of arachidonic acid with an acetylene in the 5,6 position.
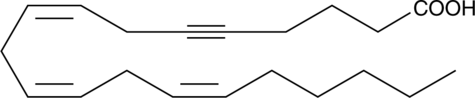
-
GC52413
5-Aminosalicylic Acid-d7
An internal standard for the quantification of 5-aminosalicylic acid
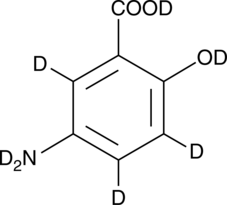
-
GC31997
5-Lipoxygenase-In-1
5-Lipoxygenase-In-1 is a 5-Lipoxygenase inhibitor extracted from patent EP 331232 A2, table 4, compound example 4.10.
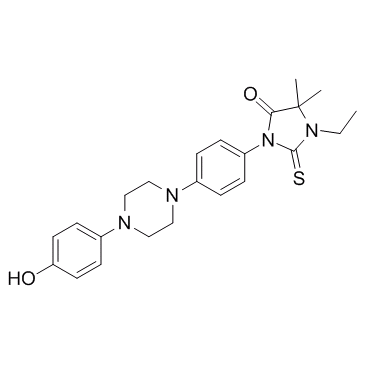
-
GC66031
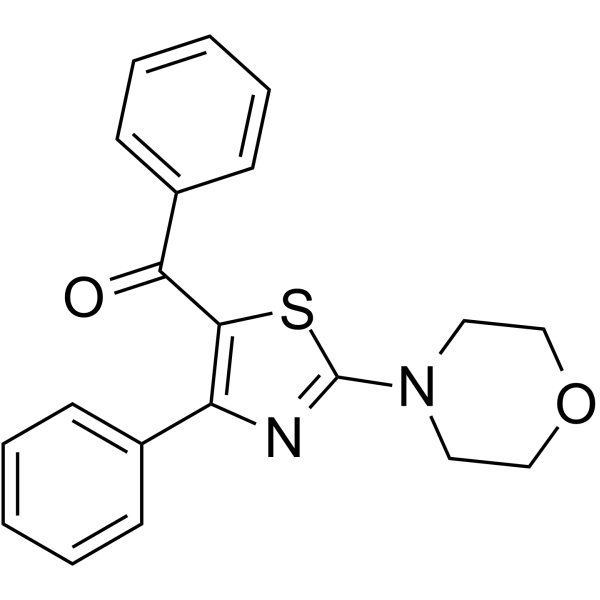
5-LOX-IN-1
5-LOX-IN-1 (compound 2b) is an inhibitor of human 5-Lipoxygenase (5-LOX) with an IC50 value of 2.3 μM. 5-LOX-IN-1 can be used for the research of inflammation.
-
GC35168
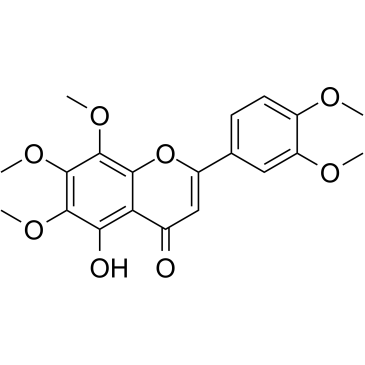
5-O-Demethylnobiletin
5-O-Demethylnobiletin (5-Demethylnobiletin), a polymethoxyflavone isolated from Sideritis tragoriganum, is a direct inhibition of 5-LOX (IC50=0.1 μM), without affecting the expression of COX-2.
-
GC40380
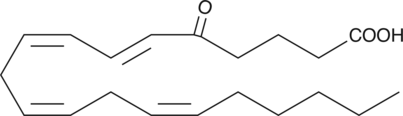
5-OxoETE
5-OxoETE is a polyunsaturated keto acid formed by the oxidation of 5-HETE in human neutrophils by a specific dehydrogenase.
-
GC41322
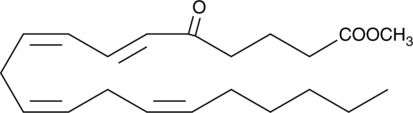
5-OxoETE methyl ester
5-OxoETE methyl ester is an esterified form of the polyunsaturated keto acid 5-oxoETE.
-
GC41424
6(S)-Lipoxin A4
The lipoxins are trihydroxy fatty acids containing a 7,9,11,13-conjugated tetraene.
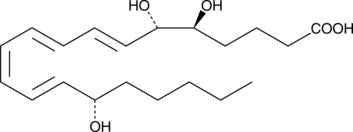
-
GC46732
7(S),17(S)-dihydroxy-8(E),10(Z),13(Z),15(E),19(Z)-Docosapentaenoic Acid
A metabolite of DPA with antiinflammatory properties
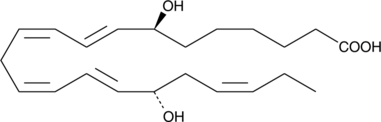
-
GC40978
7-epi Maresin 1
7-epi Maresin 1 is the inactive 7(S) epimer of Maresin 1, which contains a 7(R) hydroxyl group.
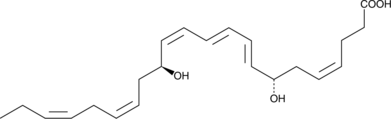
-
GC40462
8(R)-HETE
8(R)-HETE is biosynthesized by lipoxygenation of arachidonic acid in marine invertebrates such as gorgonian corals and starfish.