(±)5(6)-DiHET (Synonyms: (±)5,6-DiHETrE) |
| Catalog No.GC40270 |
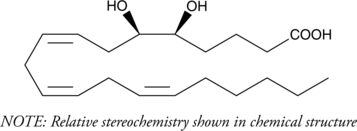
5(6)-DiHET is a fully racemic version of the enantiomeric forms biosynthesized from 5(6)-EET by epoxide hydrolases.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 213382-49-1
Sample solution is provided at 25 µL, 10mM.
5(6)-DiHET is a fully racemic version of the enantiomeric forms biosynthesized from 5(6)-EET by epoxide hydrolases. [1] 5(6)-DiHET can be used to quantify 5(6)-EET due to the conversion of 5(6)-EET to 5(6)-δ-lactone in solution. [2] 5(6)-DiHET activates large conductance calcium-activated potassium (BK) channels in smooth muscle cells from rat small coronary arteries. [3 It is a substrate for sheep seminal vesicle COX, producing 5,6-dihydroxy prostaglandin E1 and F1α metabolites in vitro. [4] 5(6)-DiHET levels decrease in plasma in a high-fat diet-induced rat model of hyperlipidemia.[5]
Reference:
[1]. Oliw, E.H., Guengerich, F.P., and Oates, J.A. Oxygenation of arachidonic acid by hepatic monooxygenases. Isolation and metabolism of four epoxide intermediates. J. Biol. Chem. 257(7), 3771-3781 (1982).
[2]. Rashid, M., Manivet, P., Nishio, H., et al. Identification of the binding sites and selectivity of sarpogrelate, a novel 5-HT2 antagonist, to human 5-HT2A, 5-HT2B and 5-HT2C receptor subtypes by molecular modeling. Life Sci. 73(2), 193-207 (2003).
[3]. Lu, T., Katakam, P.V.G., VanRollins, M., et al. Dihydroxyeicosatrienoic acids are potent activators of Ca2+-activated K+ channels in isolated rat coronary arterial myocytes. J. Physiol. 534(Pt 3), 651-667 (2001).
[4]. Oliw, E.H. Biosynthesis of 5,6-dihydroxyprostaglandin E1 and F1α from 5,6-dihydroxyeicosatrienoic acid by ram seminal vesicles. Biochim. Biophys. Acta. 795(2), 384-391 (1984).
[5]. Miao, H., Zhao, Y.-H., Vaziri, N.D., et al. Lipidomics biomarkers of diet-induced hyperlipidemia and its treatment with Poria cocos. J. Agric. Food Chem. 64(4), 969-979 (2016).
Average Rating: 5 (Based on Reviews and 33 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















