5-R-Rivaroxaban |
| Catalog No.GC11101 |
Products are for research use only. Not for human use. We do not sell to patients.
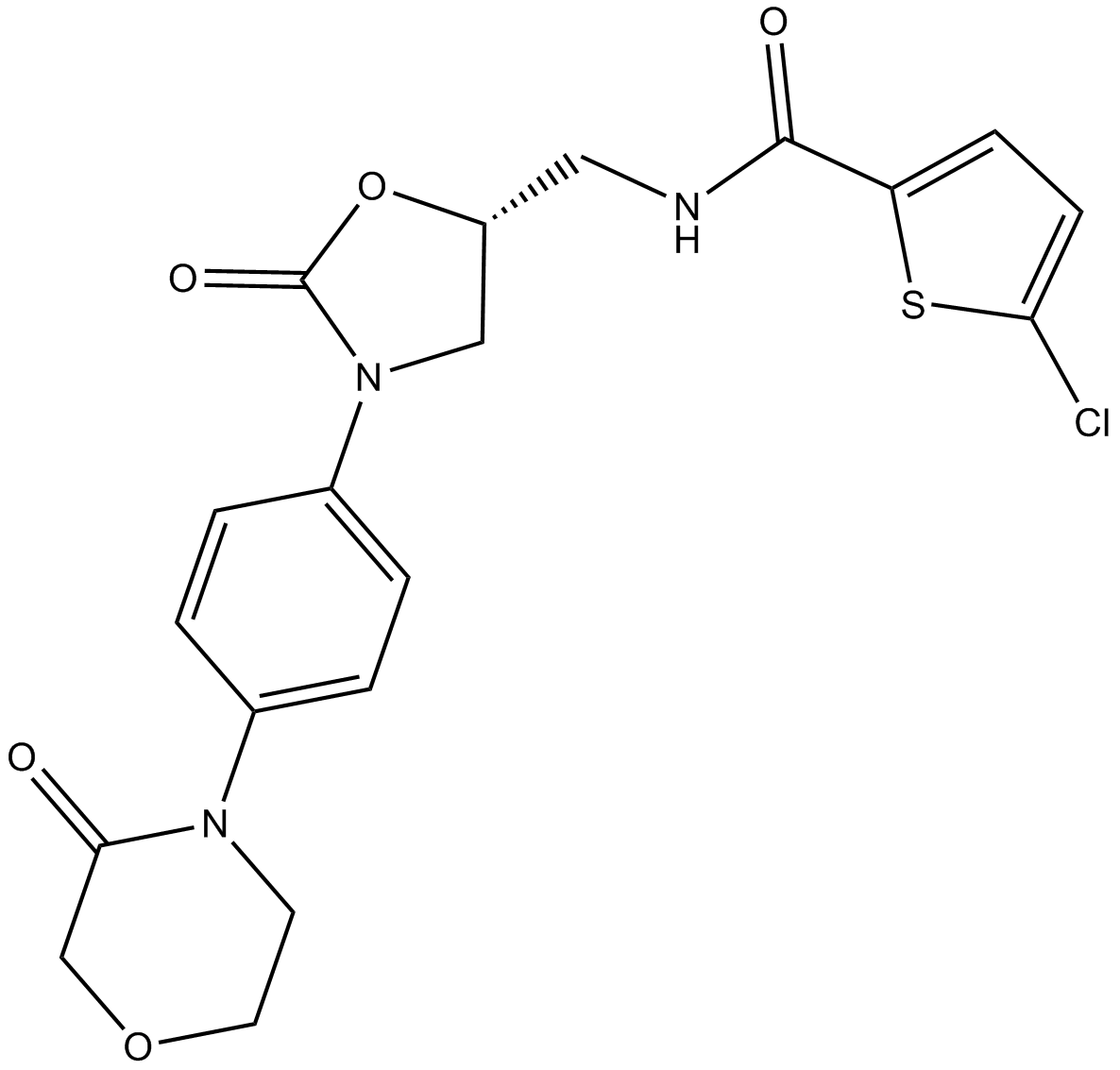
Cas No.: 865479-71-6
Sample solution is provided at 25 µL, 10mM.
5-R-Rivaroxaban is a selective inhibitor of human Factor Xa with IC50 value of 0.7 nmol/L [1].
Factor Xa is a serine endopeptidase enzyme and plays an important role in the convergence point of the intrinsic and extrinsic pathways in blood coagulation system [2].
5-R-Rivaroxaban is an oral, direct factor Xa inhibitor and the inhibition is species-dependent. When tested with purified factoe Xa from human or rabbit, 5-R-Rivaroxaban showed similar affinity with IC50 value of 0.7 nmol/L and 0.8 nmol/L, respectively, while had a IC50 value as low as 3.4 nmol/L when tested with rat factor Xa [1].
Pre-treated anaesthetised rat model with intravenous 5-R-Rivaroxaban at a dose of 2 mg/kg, and after bleeding initiated intravenous treated with rFVIIa (100/400 μg/kg), PCC (25/50 U/kg) or aPCC (50/100 U/kg), the result showed that 5-R-Rivaroxaban pre-treatment significantly shorten bleeding time and clotting time compared with 5-R-Rivaroxaban alone treated group [2]. Similar results were obtained when tested with rabbit model [1].
It has been reported that 5-R-Rivaroxaban is a promising drug for atrial fibrillation, venous thromboembolism or thromboembolic disorders in clinic [3] [4] [1].
References:
[1]. Perzborn, E., et al., Rivaroxaban: a new oral factor Xa inhibitor. Arterioscler Thromb Vasc Biol, 2010. 30(3): p. 376-81.
[2]. Perzborn, E., et al., Reversal of rivaroxaban anticoagulation by haemostatic agents in rats and primates. Thromb Haemost, 2013. 110(1): p. 162-72.
[3]. Beyer-Westendorf, J., et al., Efficacy and safety of rivaroxaban or fondaparinux thromboprophylaxis in major orthopedic surgery: findings from the ORTHO-TEP registry. J Thromb Haemost, 2012. 10(10): p. 2045-52.
[4]. Palareti, G., et al., Clinical management of rivaroxaban-treated patients. Expert Opin Pharmacother, 2013. 14(5): p. 655-67.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















