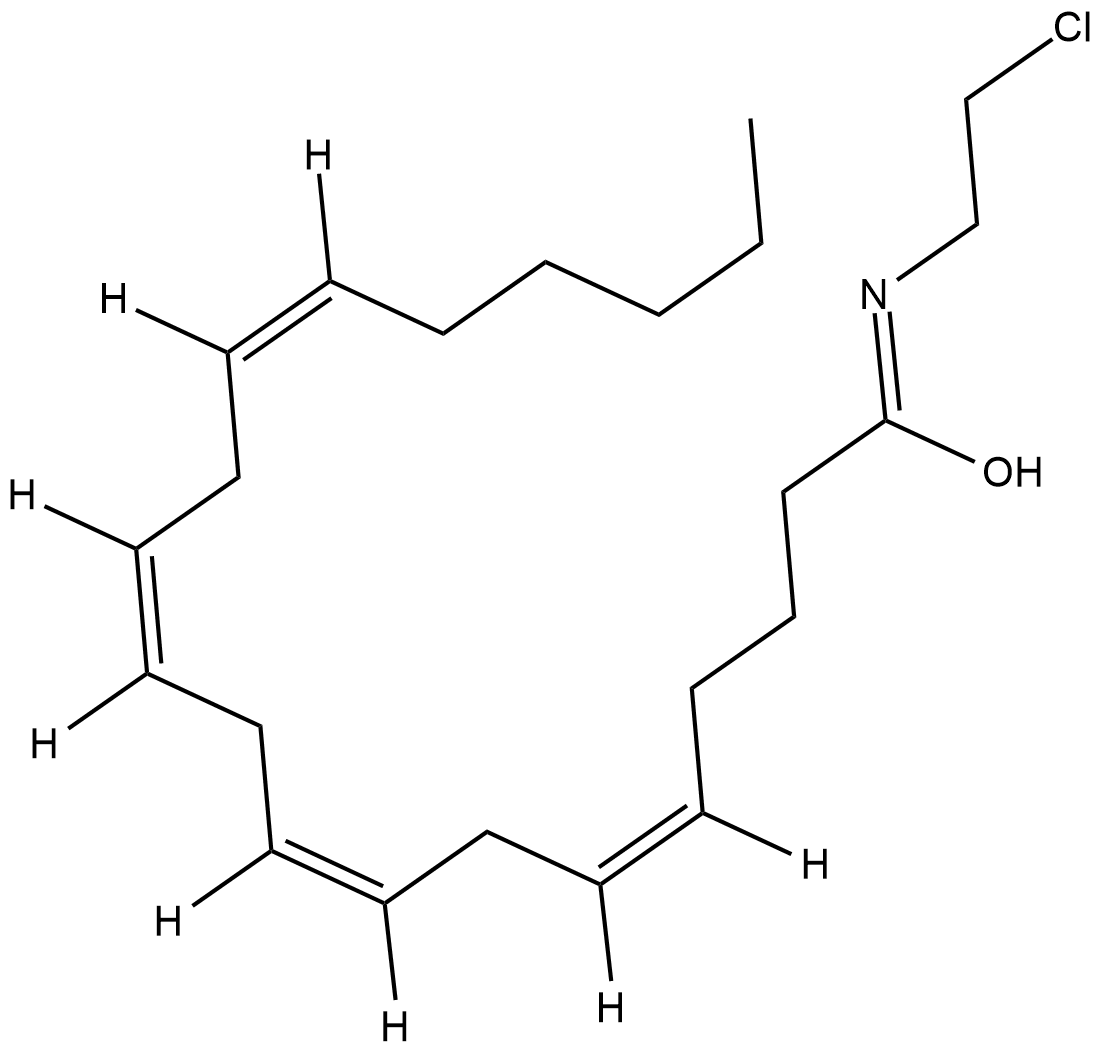
Arachidonoyl 2'-Chloroethylamide (Synonyms: ACEA, 2'chloroAEA) |
| Catalog No.GC18435 |
A potent and selective CB1 receptor agonist
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 220556-69-4
Sample solution is provided at 25 µL, 10mM.
Arachidonoyl 2-chloroethylamide (ACEA) is a potent and selective cannabinoid (CB) receptor 1 agonist with Ki values of 1.4 and >2,000 nM for CB1 and CB2 receptors, respectively. In whole animal experiments, ACEA induces hypothermia in mice with the same efficacy as arachidonoyl ethanolamide , in spite of its higher affinity for the CB1 receptor. These data have been interpreted to indicate that ACEA may be a substrate for fatty acid amide hydrolase (FAAH), and thus only transiently available in whole animal experiments.
References:
[1].Pertwee, R.G. Pharmacology of cannabinoid receptor ligands Curr. Med. Chem. 6(8), 635-664 (1999).
[2].Hillard, C.J., Manna, S., Greenberg, M.J., et al. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1) J. Pharmacol. Exp. Ther. 289(3), 1427-1433 (1999).
Average Rating: 5 (Based on Reviews and 24 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















