AZD8931 (Sapitinib) (Synonyms: Sapitinib) |
| Catalog No.GC13761 |
AZD8931 (Sapitinib) (AZD-8931) is a reversible, ATP competitive EGFR inhibitor of with IC50s of 4, 3 and 4 nM for EGFR, ErbB2 and ErbB3 in cells, respectively.
Products are for research use only. Not for human use. We do not sell to patients.
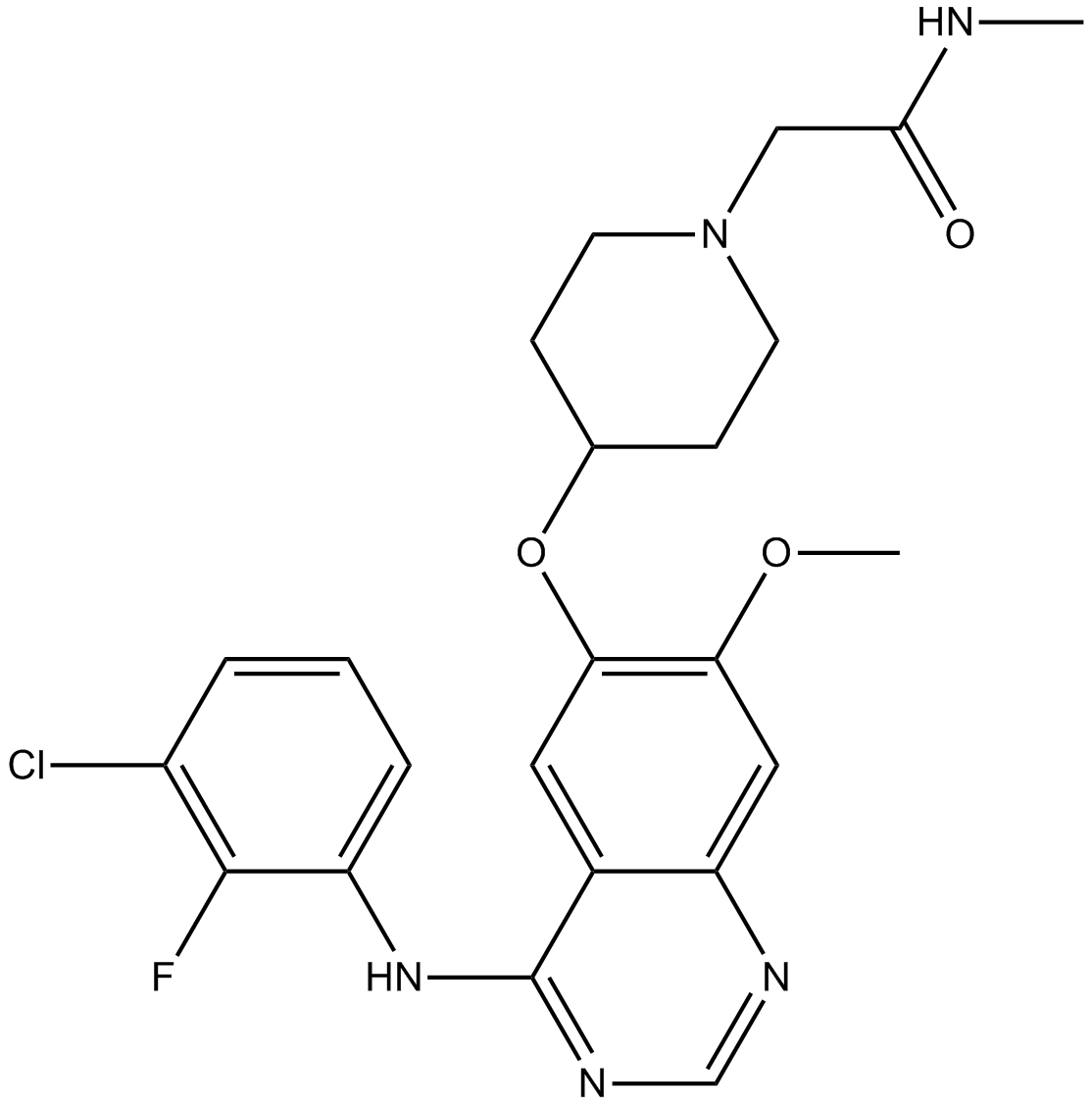
Cas No.: 848942-61-0
Sample solution is provided at 25 µL, 10mM.
Description:
IC50: AZD8931 showed equipotent, reversible inhibition of EGFR (IC50, 4 nmol/L), erbB2 (IC50, 3 nmol/L), and erbB3 (IC50, 4 nmol/L) phosphorylation in cells
The erbB receptor family is composed of four related receptor tyrosine kinases [epidermal growth factor receptor (EGFR, erbB1), erbB2 (human epidermal growth factor receptor 2, HER2), erbB3 (HER3), and erbB4 (HER4)]. AZD8931 is a novel small-molecule equipotent inhibitor of EGFR, HER2, and HER3 signaling.
In vitro: In a previous in vitro study, the authors investigated the antitumor activity of AZD8931 alone or in combination with paclitaxel using preclinical models of EGFR-overexpressed and HER2 non-amplified IBC cells. Results showed that AZD8931 significantly suppressed cell growth of IBC cells and induced apoptosis of human IBC cells in vitro [2].
In vivo: In a previous study, the authors investigated the antitumor activity of AZD8931 alone or in combination with paclitaxel IBC cells were orthotopically transplanted into the mammary fat pads of immunodeficient mice. AZD8931 was given by daily oral gavage at doses of 25 mg/kg, 5 days/week for 4 weeks. Paclitaxel was subcutaneously injected twice weekly. Results showed that AZD8931 monotherapy inhibited xenograft growth and the combination of paclitaxel + AZD8931 was demonstrably more effective than paclitaxel or AZD8931 alone treatment at delaying tumor growth in vivo in orthotopic IBC models. These results suggest that AZD8931 may provide a novel therapeutic strategy for the treatment of IBC patients with HER2 non-amplified tumors [2].
Clinical trial: A phase I/II clinical trial is conducted to determine if AZD8931 can improve the efficacy of standard chemotherapy for the treatment of advanced breast cancer. This study will be conducted in 2 parts: the first part (phase I) will determine a dose of AZD8931 that can be safely administered with paclitaxel chemotherapy. The second part (phase II) will determine the efficacy and safety of AZD8931 in combination with paclitaxel chemotherapy in breast cancer (https://clinicaltrials.gov/ct2/show/NCT00900627).
References:
[1] Hickinson DM, Klinowska T, Speake G, Vincent J, Trigwell C, Anderton J, Beck S, Marshall G, Davenport S, Callis R, Mills E, Grosios K, Smith P, Barlaam B, Wilkinson RW, Ogilvie D. AZD8931, an equipotent, reversible inhibitor of signaling by epidermal growth factor receptor, ERBB2 (HER2), and ERBB3: a unique agent for simultaneous ERBB receptor blockade in cancer. Clin Cancer Res. 2010;16(4):1159-69.
[2] Mu Z, Klinowska T, Dong X, Foster E, Womack C, Fernandez SV, Cristofanilli M. AZD8931, an equipotent, reversible inhibitor of signaling by epidermal growth factor receptor (EGFR), HER2, and HER3: preclinical activity in HER2 non-amplified inflammatory breast cancer models. J Exp Clin Cancer Res. doi: 10.1186/1756-9966-33-47.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















