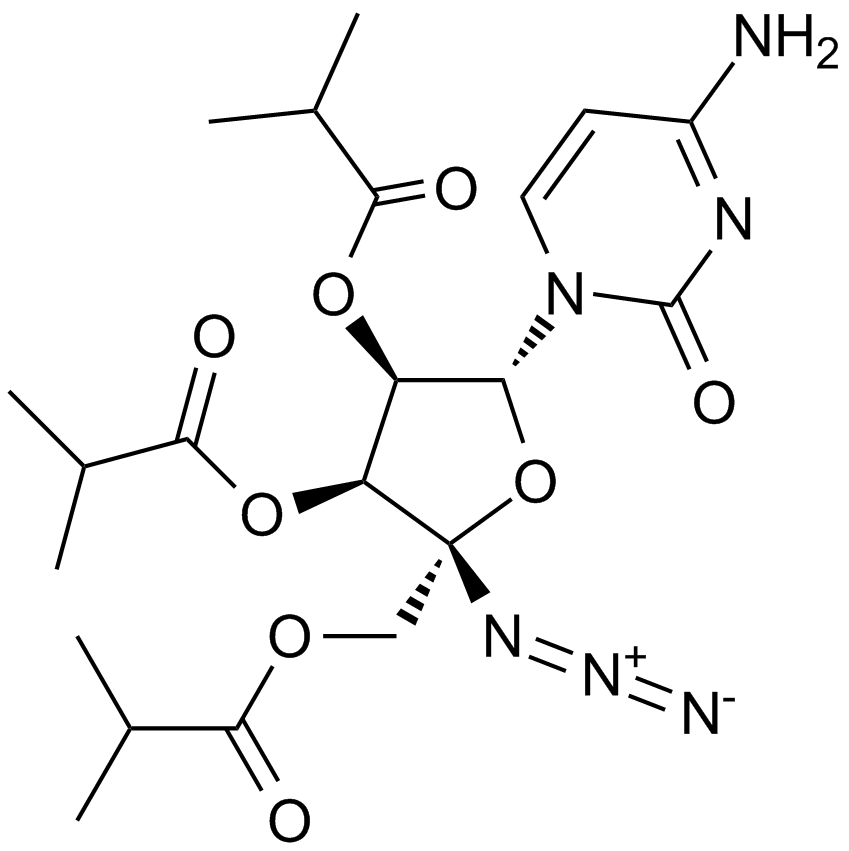
Balapiravir |
| Catalog No.GC10930 |
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 690270-29-2
Sample solution is provided at 25 µL, 10mM.
Balapiravir (R1626, Ro 4588161) is the prodrug of a nucleoside analogue inhibitor of the hepatitis C virus (HCV) RNA-dependent RNA polymerase (R1479, RG1479).IC50 Value: Target: HCVBalapiravir(R-1626; R 1626; Ro 4588161) is useful for Anti HCV. Balapiravir (R1626) is the tri-isobutyrate ester prodrug of R1479 under clinical development to improve exposure of R1479 upon oral administration. Balapiravir was discontinued for safety reasons in 28-36% of patients (most often for lymphopenia) and the percentage of patients with serious adverse events (especially hematological, infection, ocular events) was dose related. Serious hematological adverse events (particularly neutropenia, lymphopenia) were more common in balapiravir recipients. Two deaths in the balapiravir/peginterferon alfa-2a/ribavirin combination groups were considered possibly related to study medication.
References:
[1]. Nguyen NM, et al. A randomized, double-blind placebo controlled trial of balapiravir, a polymerase inhibitor, in adult dengue patients. J Infect Dis. 2013 May 1;207(9):1442-1450.
[2]. Nelson DR, et al. Balapiravir plus peginterferon alfa-2a (40KD)/ribavirin in a randomized trial of hepatitis C genotype 1 patients.Ann Hepatol. 2012 Jan-Feb;11(1):15-31.
[3]. Li F, et al. Chemical stability of 4'-azidocytidine and its prodrug balapiravir.Drug Dev Ind Pharm. 2010 Apr;36(4):413-20.
[4]. kros PJ, et al. R1626 plus peginterferon Alfa-2a provides potent suppression of hepatitis C virus RNA and significant antiviral synergy in combination with ribavirin.Hepatology. 2008 Aug;48(2):385-97.
[5]. Roberts SK, et al. Robust antiviral activity of R1626, a novel nucleoside analog: a randomized, placebo-controlled study in patients with chronic hepatitis C.Hepatology. 2008 Aug;48(2):398-406.
[6]. Chen YL, et al. Activation of peripheral blood mononuclear cells by dengue virus infection depotentiates balapiravir. J Virol. 2014 Feb;88(3):1740-1747.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















