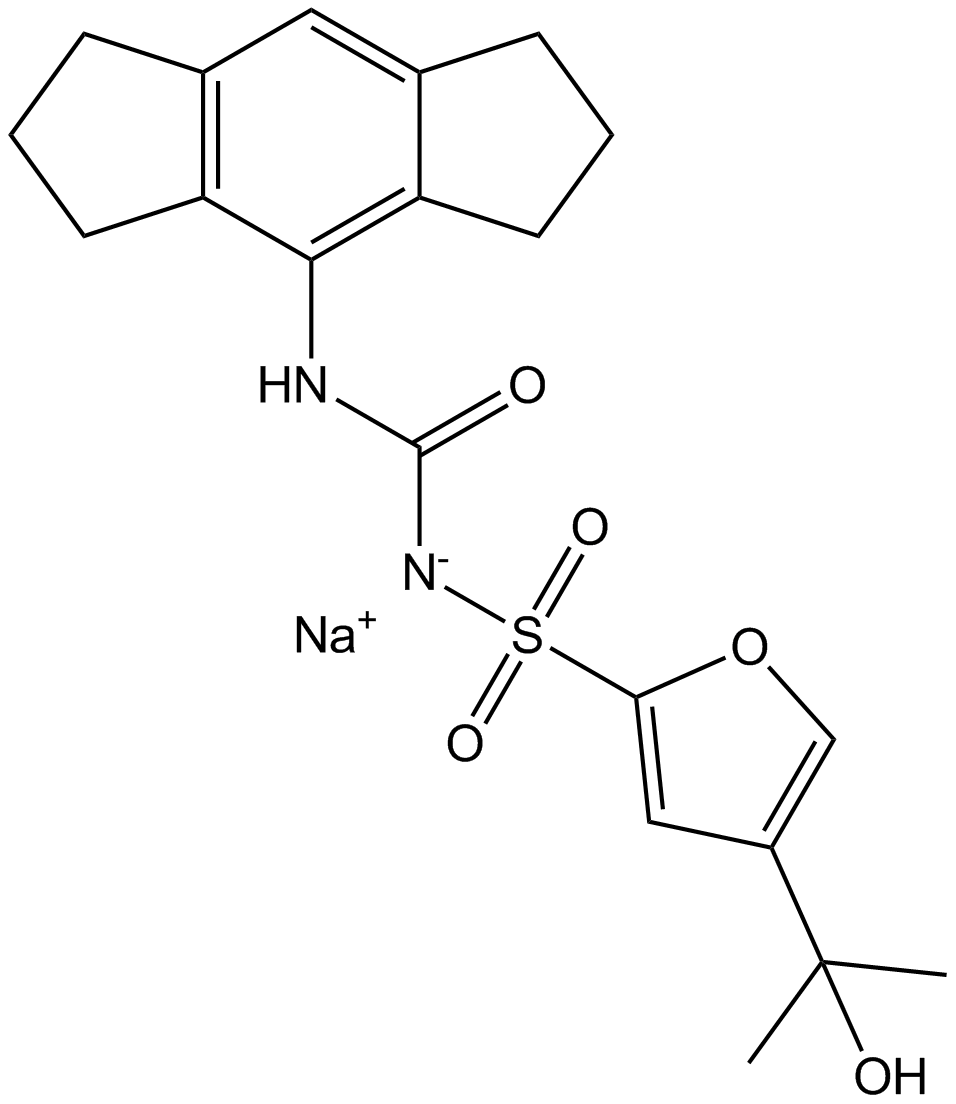
MCC950 Sodium
CP-456773, cytokine release inhibitory drug-3 CRID3, MCC950 sodium and MCC950, all are the short forms forN-(1,2,3,5,6,7-hexahydro-S-indacen-4-ylcarbamoyl)-4-(2-hydroxy-2-propanyl)-2-furansulfonamide. Chemically, it is a derivative of diaryl sulfonylurea derivative. It is a small molecule. Its molecular structure is diagrammatically depicted in Figure 1. The roadmap for its synthesis is documented in the scientific literature in detail.
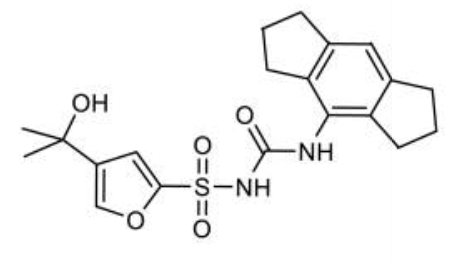
Figure 1 The Molecular Structure of MCC950
MCC950 has an anti-inflammatory effect as it effectively inhibits NOD-Like Receptor pyrin domain-containing Protein 3 (NLRP3), which actively participates in the activation of NLRP3 inflammasome along with the other specialized proteins, which in turn activates the whole inflammation process leading to a specialized form of apoptosis/ programmed cell death that is known as pyroptosis, a landmark in a number of pathological conditions, for example, Cryopyrin Associated Periodic Syndromes (CAPS), multiple sclerosis, type 2 diabetes, atherosclerosis. Besides these, NLRP3 has also prominent role in different types of diseases, for example, metabolic ones, obesity and gout; neural diseases, Alzheimer’s disease, Parkinson’s disease; non-alcoholic fatty liver disease; lung diseases, asthma and fibrosis; silicosis; kidney diseases; bone dieases, rheumatoid arthritis, osteoarthritis, Crohn’s disease; cardiovascular diseases, coronary artery diseases. Thus, MCC950 can be used as a therapeutic agent as it possesses ideal pharmacokinetic and pharmacodynamic properties in addition to selectivity, especially against those diseases in which NLRP3 is actively involved. Moreover, as a high level of safety has been associated with using it, as indicated in the several clinical trials. Using MCC950, is especially important against the pathologies involving a defective immune system, i.e., autoinflammatory and autoimmune ones. As in these pathologies, body’s immune system is abnormally responding to body’s normal proteins and tissues.
Apart from being used as a therapeutic agent, MCC950 can be useful in the research, studying the roles of NLRP3 in the cell and its potential pharmaceutical targets.
It is highly specific in its action as evident from Figure 2. Moreover, it is effective in the range of nano molars with its half-maximal inhibitory concentration (IC50) of MCC950 for the Interleukin-1 beta (IL-1β) release. Keep in mind that the release of Interleukin-1 beta (IL-1β) is the downstream event of the activation of NLRP3.
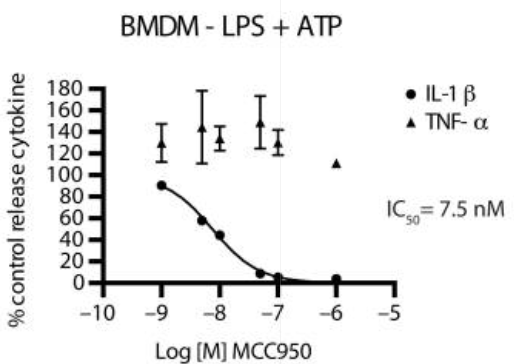
Figure 2 The release of Interleukin-1 beta (IL-1β) as indicated by circles and Tumor Necrosis Factor-α (TNF-α) as indicated by triangle in the mouse bone marrow derived macrophages (BMDM) treated with MCC950. The release of the former decreased and, is stopped completely at 7 nM, with the IC50 of 7.5 nM, while the release of the latter is nearly remains unchanged. This shows that MCC950 selectively targets the Interleukin-1 beta (IL-1β) release.
NLRP3 protein is the functional protein of the innate immune system (Tapia-Abellán et al., 2019) that contains three domains, namely: pyrin domain (PYD) at N-terminal/ amino terminal, leucine-rich repeat domain (LRR domain) at C-terminal/ carboxy terminal while NACHT domain in the middle (Freeman and Swartz, 2020). The positions of all these three domains in the NLRP3 protein is diagrammatically illustrated in Figure 3. NLRP3 resides in the cytosol, thus it is a cytosolic protein.

Figure 3 Three Domains of the NLRP3 protein, that is the target of MCC950
The expression of NLRP3, that is also called priming and its subsequent activation are the two phases that lead to the functional NLRP3 in the macrophages, the specialized cells. The expression of NLRP3 and pro-IL-1β in the macrophages, is upregulated by the agonist of Toll-like receptor 4 (TLR4). Along with this, some of the post-translational modifications (PTMs) of NLRP3, like its phosphorylation and ubiquitination, are also very important to the successful formation of a functional NLRP3 inflammasome. Interestingly, MCC950 does not inhibit this first step, i.e., priming, as it does not interfere with the Toll-like receptor 4 (TLR4) in any way.
The activation of NLRP3 is triggered by several factors, i.e., danger-associated molecular patterns (DAMPs), like silica and uric acid crystals; pathogen-associated molecular patterns (PAMPs), like surface proteins of the pathogens, environmental and host factors. Therefore, it is termed as a sensor molecule in the cell. Upon its activation, it gets oligomerized. Then, it gets associated with apoptosis associated speck-like protein containing a CARD, ASC via its pyrin domain (PYD). Then, CARD domain of ASC attracts the CARD domain of pro-caspase-1, forming NLRP3 inflammasome, which is a multimeric protein (Tapia-Abellán et al., 2019). This multimeric protein is actually a protein complex, i.e., NLRP3–ASC–pro-caspase-1 complex. In this complex, NLRP3 acts as a sensor, ASC acts as an adaptor while caspase-1 acts as an effector. This generates a signaling cascade in the cell, that leads to the activation of caspase-1. Activated caspase-1 activates the cytokine proteins via cleavage of pro-IL-1β and pro-IL-18, which promotes the inflammation process and leads to the pyroptosis via gasdermin D cleavage, that separates its N-terminal domain, also known as Active N-terminus that eventually causes pore formation in the plasma membrane of the cells. The most prominent cytokines are Interleukin-1 beta (IL-1β) and Interleukin-18 (IL-18).
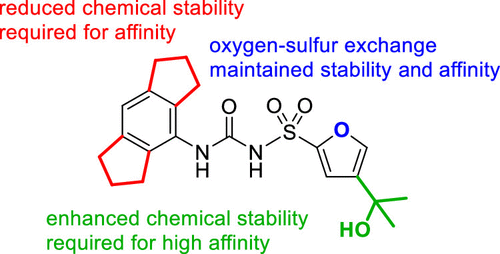
Figure 4 A deeper looker at the Structure-Stability Relationships within MCC950; the two rings in red and the three branches reduce and enhance the stability which is required for the affinity. While the blue oxygen is the site which keeps a balance the chemical stability as well as chemical affinity
It has been scientifically shown that MCC950 inhibits NLRP3 by directly non covalently interacting with the NLRP3; more specifically with its middle domain NACHT domain and even more specifically with its Walker B ATP-hydrolysis motif. This site was responsible for the hydrolysis of ATP, a key step in the activation of NLRP3. As at this motif, MCC950 is interacting instead of ATP, the hydrolysis of ATP is prevented. A research study aimed at to study the structure of this NACHT domain of NLRP3 protein. It has been demonstrated that this particular domain is composed of four subdomains; and interestingly enough, these subdomains are arranged in such a configuration that a pocket is formed where inhibiting molecule can interact and bind. That enables the inhibitor to act as an intramolecular glue, and render the NLRP3 inactive . This pocket in the NACHT domain along with the inhibiting molecule is demonstrated in Figure 5. The key molecular features of MCC950 involved in this interaction are highlighted in Figure 4. It has also been experimentally demonstrated that MCC950 brings the conformational changes in the NLRP3 structure, i.e., it closes it and thereby render it inactive.
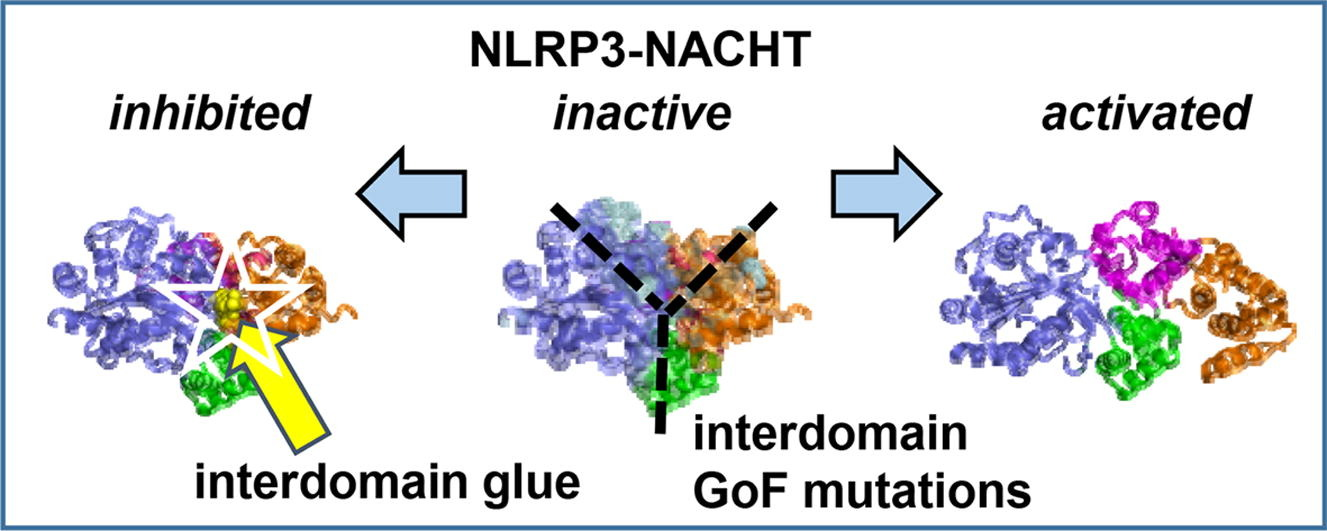
Figure 5 The middle domain of NLRP3 protein; NACHT domain with its four subdomains, in purple, orange, green and magenta. The activation and inactivation of NLRP3 is determined by the pocket (structural feature) which interacts with the inhibiting molecule shown in yellow and indicated by a star.
All the molecular events discussed so far are summarized in Figure 6.
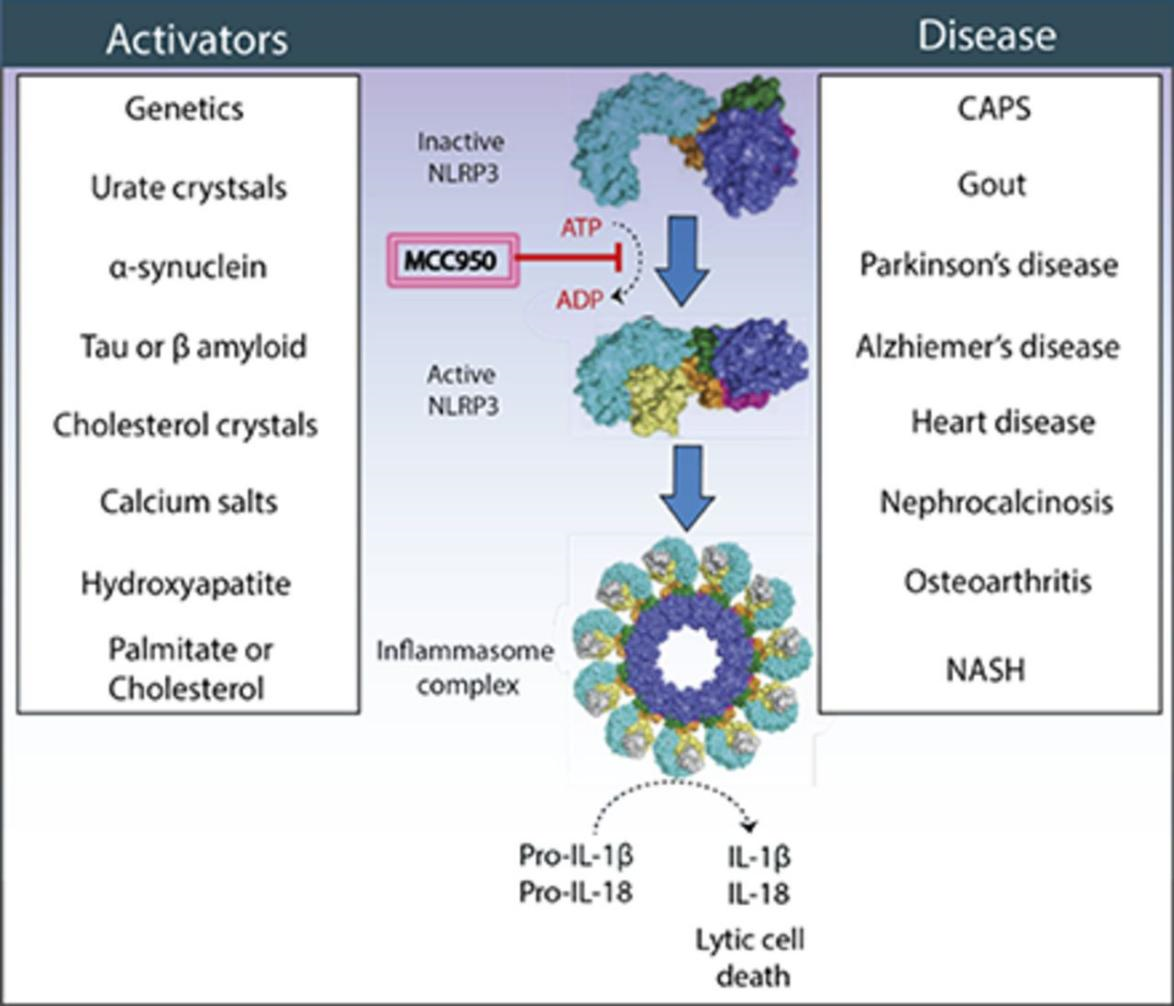
Figure 6 The Molecular Events involved in the activation and inhibition of NLRP3












Comments