Mitomycin C
Mitomycin C is also known as 7-amino-9α-methoxymitosane (Carlos de Oliveira and Wilson, 2020). Chemically, Mitomycin C is a small molecule. Its molecular structure is diagrammatically shown in Figure 1.
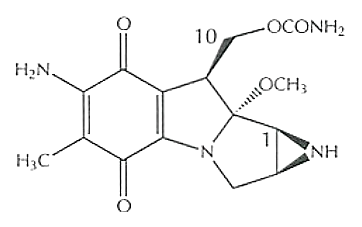
Figure 1 The Molecular Structure of Mitomycin, in which carbon positions, i.e., C1 and C10 are mentioned as both of them act as alkylating centers in the DNA cross-linking, in a sequential manner.
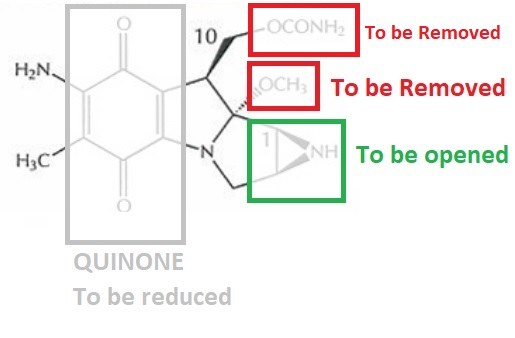
Figure 2 The Structural Features in the native structure of Mitomycin C, which must be either chemically altered or removed to cause cross linking in the DNA double helix .
Mitomycin C acts as a cross linking agent for the DNA double helix. But it cannot cross link DNA in its native molecular form (as shown in Figure 1) rather it has to be either chemically or enzymatically reduced to cross link the DNA strands. It has a quinone ring (as indicated within a grey box in Figure 2), which has to be reduced followed by a number of chemical transformations that eventually leads to the removal of OCH3 (as indicated within a red box in Figure 2) and opening of aziridine ring structure (as indicated within a green box in Figure 2). After these molecular changes, a highly reactive and unstable molecular species is formed, i.e., vinylogous quinone methide. These molecular changes along with the final active species are diagrammatically illustrated in Figure 3. As vinylogous quinone methide contains a carbon at C1 position, which is now exposed (indicated in Figure 3). It is exactly the site where DNA strand at the guanine (nitrogenous base) will get attached via first Alkylation.
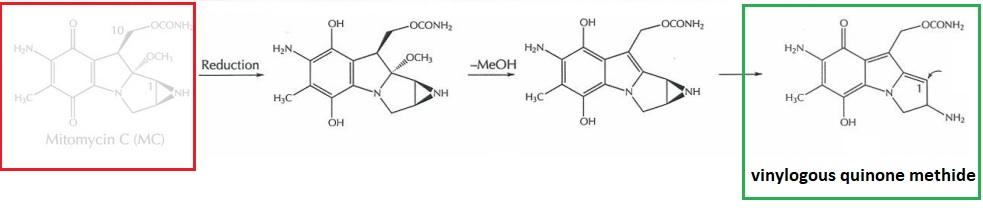
Figure 3 The Chemical transformations which are involved in the conversion of inactive Mitomycin C (red box) into a highly active Vinylogous Quinone Methide (green box)
Second Alkylation step is optional. That means, it may take place or not. After first Alkylation, OCONH2 is also removed, thereby carbon at C10 position get exposed and acts an alkylating center. At this very carbon position, either same or different strand of one DNA double helix or another at the guanine (nitrogenous base), gets attached via a subsequent second Alkylation. There two consecutive Alkylation steps are diagrammatically depicted in Figure 4.
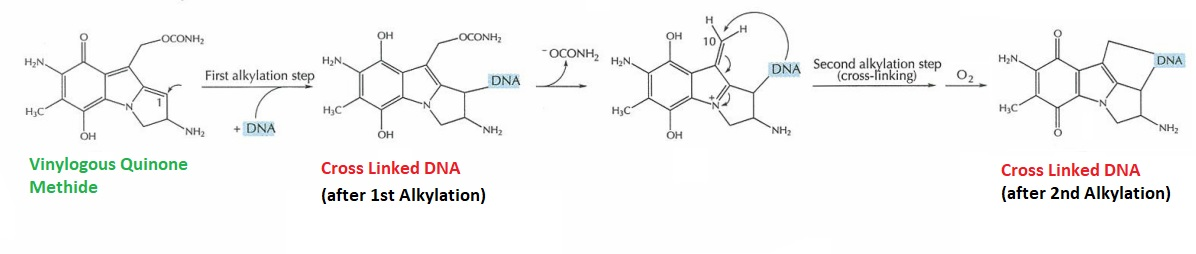
Figure 4 Cross-Linked DNA formed by Vinylogous Quinone Methide (Simplified) (Tomasz, 1995)
All the possible (versions) products/ adducts of these DNA cross linkage, which are isolated in a scientific study, are diagrammatically illustrated in Figure 5 (after Monofunctional Activation/ one Alkylation step) and Figure 6 (after Bifunctional Activation/ two Alkylation steps).
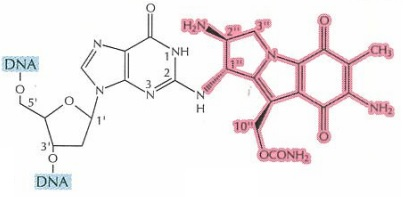
Figure 5 Single product type formed after Monofunctional Activation of Mitomycin C. The cross-linked DNA is highlighted in blue, while Mitomycin C in pink (Tomasz, 1995)
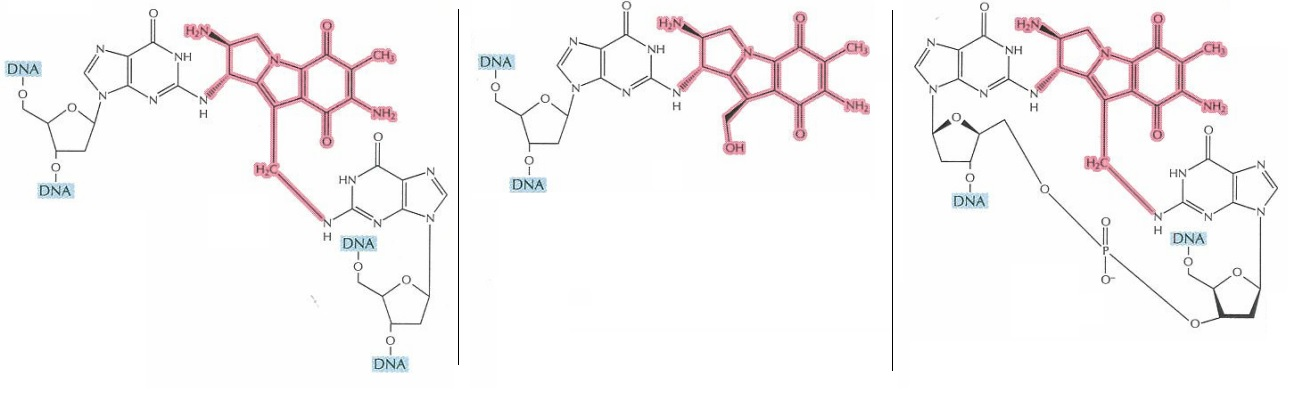
Figure 6 Three distinct products can potentially be formed after Bifunctional Activation of Mitomycin C. The cross-linked DNA is highlighted in blue, while Mitomycin C in pink (Tomasz, 1995). Only in the right most molecule, two DNA stands are covalently cross linked. On the other hand, rest of two molecules has the “limpet attachment”, which also affect the DNA replication and cell multiplication.
DNA cross linkage is extremely deadly for the bacteria, even one such cross linkage in an entire genome has the ability to cause death in that bacterium. Hence, Mitomycin C has the antibiotic effect owing to its ability to cross link DNA.
Another thing is that DNA double helix is opened for the DNA duplication by the activity of a helicase enzyme. As normally, there is no chemical bind between the two DNA strands/ molecules, cross linking creates a covalent bond between these DNA strands/ molecules. As a result, helicase cannot open these DNA strands/ molecules because between them is not only the hydrogen bonding but also the covalent bonding. This covalent bonding prevents the separation of DNA strands/ molecules, which is required for the DNA replication specifically or cell division generally. Thus, the multiplication of these cells is stopped.
As Mitomycin C can inhibit the proliferation of the fibroblasts irrespective of their cell cycle stage, thus, fibrosis, inflammatory responses (thus prevents the scar formation) and growth of new blood vessels are automatically retarded (Zhang et al., 2021). It also significantly increases the probability of the formation of a functional bleb, which is therapeutically beneficial. This effect of Mitomycin C has been clinically exploited as it is used in trabeculectomy, which is a special type of eye surgery/ procedure. (Swogger et al., 2020).
Mitomycin C also has a cytotoxic effect against tumors. It is graphically shown against the cancerous cells of bladder in Figure 7. As evident from Figure 7, this effect is dose dependent in nature. Besides this, also Mitomycin C stops the progression of cell cycle in these cells. Thus, causing a phenomenon called as cell cycle arrest. As evident from Figure 8, most of the cancerous cells are prevented from entering the synthesis (S) phase of the cell cycle. This is done as a function of concentration and indicated by the decreasing heights of the red bar graphs as the Mitomycin C concentration is increased. On the other hand, a majority of the cancerous cells are kept held/ arrested at the G2/ M phase. This is particularly evident from the increased heights of green bar graph in Figure 8.
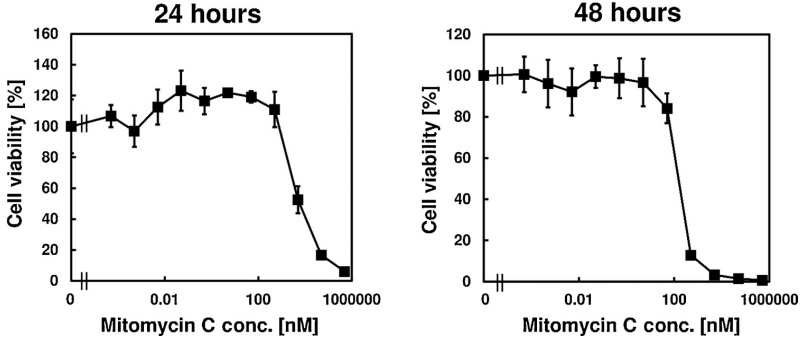
Figure 7 The Cytotoxic Effect of Mitomycin C on the cancerous cell of bladder. All the cancerous cell were died at the concentration of 1 mM (in left graph) and 0.05 M (in right graph) Mitomycin C for 24 hrs. and 48 hrs., respectively (Nakayama et al., 2020).
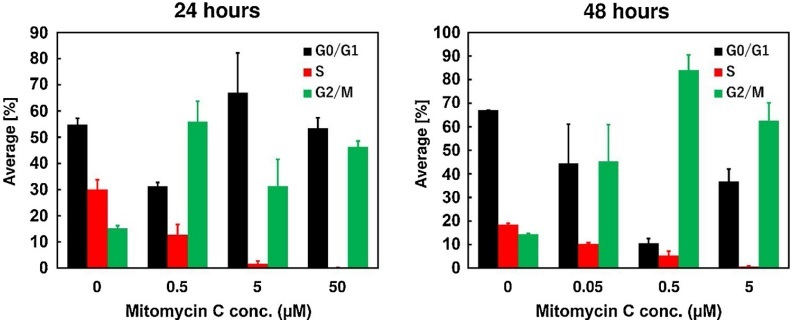
Figure 8 Mitomycin causes the cell cycle arrest in the cancerous cells of bladder mainly at the G2 / M stage.
At a particular concentration of Mitomycin C, an intermediate state of the cancer cells can be attained; which is partially dead and partially arrested. This dormant state is diagrammatically presented in Figure 9.
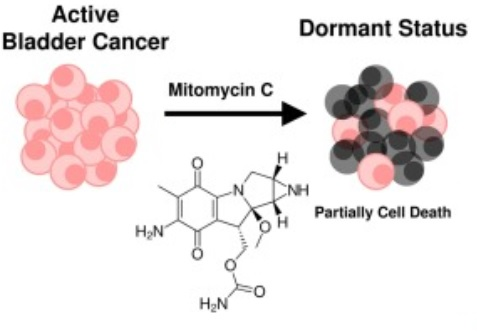
Figure 9 The dormant state of the cancer cells, attained via treating them with Mitomycin C.
PEPT1, ABCB6, ABCG2 are the protein, involved in the intake of 5-Aminolevulinic acid (ALA), transport and export of Protoporphyrin IX (PpIX) within the cancerous bladder cells. The expression of all of these proteins is upregulated as the concerned genes are upregulated by Mitomycin C, and thereby the biosynthesis of Protoporphyrin IX (PpIX) is upregulated. Not only the Protoporphyrin IX (PpIX) concentration is found to be increased in a cell but also in the extracellular space due to Mitomycin C.
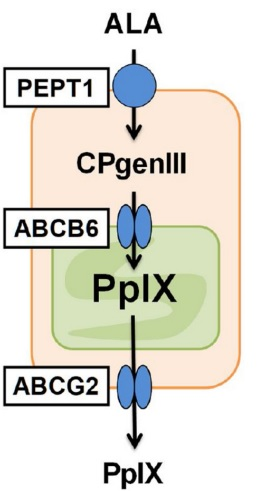
Figure 10 The transporter proteins, i.e., PEPT1, ABCB6, ABCG2, which are involved in the biosynthesis of Protoporphyrin IX (PpIX) from 5-Aminolevulinic acid (ALA) within the cancerous cells of the bladder.
In that research study, it has been shown that the suppression of the tumor growth and their death were more effective in those cells, which are treated with Mitomycin C and ALA-photodynamic therapy (ALA-PDT). Mitomycin C enhances the therapeutic effect of ALA-PDT against various neoplasms, for example, gastric cancer, bladder cancer, prostate cancer, ovarian carcinoma.
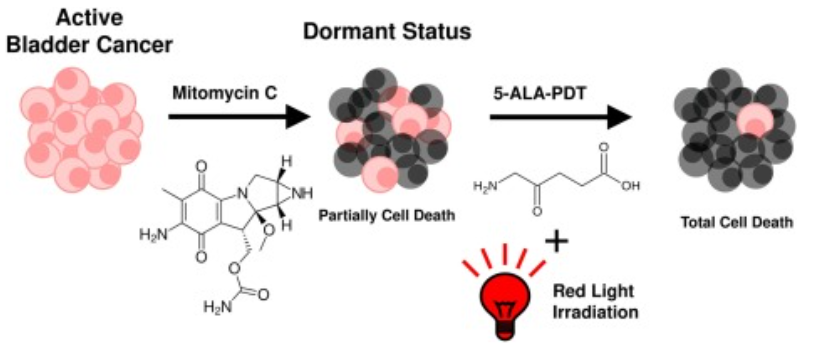
Figure 11 The cell death of Cancerous cells/ tumors is effectively attained by the combined sequential action of Mitomycin C and ALA-PDT. The alive/ active cells are shown in pink, while dead/ inactive in grey.













Comments