Staurosporine
Staurosporine (Str) is an alkaloid, which has a complex molecular structure comprising of nine fused rings. Its structure is diagrammatically depicted in Figure 1. It is primarily classified as a derivative of Indolocarbazole (Toledo and Lydon).
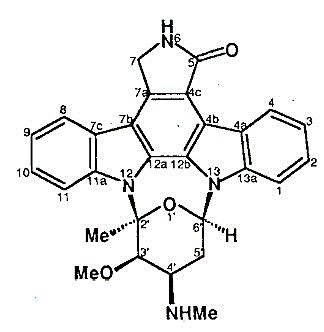
Figure 1 The Molecular Structure Staurosporine (Str), as determined by the x-rays analysis
It is a hydrophobic in nature, therefore, can potentially bind to a number of enzymes through hydrophobic interactions. It is capable of inhibiting many different protein kinases, to name a few, Protein Kinase B (PKB), Protein Kinase C (PKC), Tyrosine Kinase, Myosin Light Chain (MLC) Kinase, cdc2 Kinase and IkappaB kinase (shortened as IkB kinase or IKK). Protein kinases are majorly involved in transducing of the signal. Its dysregulation is prominent in various cancers and immune system disorders. Thus, it is non-specific (or non-selective) in its inhibitory action. It is further graphically explained in Figure 2 (Omura et al., 1995).
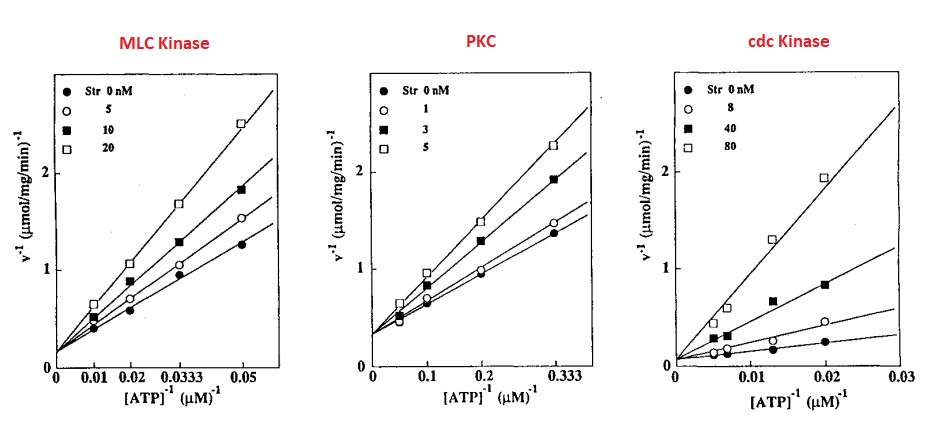
Figure 2 The non-specific inhibitory action of the Staurosporine (Str) is indicated by the approximate same level of inhibition of Myosin Light Chain (MLC) Kinase, Protein Kinase C (PKC) and cdc2 Kinase for all the given concentrations of Staurosporine (Str). This inhibitory action competes with the availability of ATP. Moreover, it is directly proportion to the concentration of Staurosporine (Str).
Protein Kinase B (PKB) phosphorylates the caspase-9 and render it inactive. Besides this, it also phosphorylates Bcl-2-associated death promoter (BAD) and render it in as anti-apoptotic. In this case the release of Cytochrome C is inhibited. IKK regulates the activation of Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which promotes the synthesis of proteins primarily having anti-apoptotic properties.
Usually, Apoptosis is a desired event for the cancerous cell/ tumors irrespective of the cell type. Apoptosis can be induced by using Protein Kinase inhibitors; in this case, Staurosporine (Str). But, the exact mechanism of the Apoptosis induction by any compound must be known as it is critical to develop effective anti-cancer therapies. In Staurosporine (Str)-induced Apoptosis, cytochrome C is released and Caspases are activated. It is assumed that this apoptosis is independent of the nuclear signalling and thus can be processed in the cells deprived of nucleus (Stepczynska et al., 2001).
Staurosporine (Str) can invariably cause Apoptosis in all the mammalian cells. The underlying mechanism of and involved pathways in the Staurosporine (Str)-induced Apoptosis is a bit unique and relatively more complex, when compared to the other traditionally available anti-cancer drugs. This subtle difference is shown as the Caspase Activation was inhibited in the anti-cancer treated-cells expressing catalytically inactive form of caspase-9, while Caspase Activation was not inhibited Staurosporine (Str) treated-cells expressing catalytically inactive form of caspase-9. This difference is also reconfirmed in other type of cells having a mutant construct of caspase-9. In that case, caspase-8 activation was inhibited instead of caspase-9, which is a downstream step of caspase-9 activation. This is significantly important and useful as by combining Staurosporine (Str) with the traditional anti-cancer drugs, the resistance shown by the cancerous cell to the anti-cancer drugs can be controlled and mitigated.
Interestingly, Staurosporine (Str) is also found to induce Apoptosis in those cancerous cells, which have become resistant to traditionally used anti-cancer drugs, namely JE6.1 and JM319 cells. The Caspase Activity, which is a popular landmark of Apoptosis, is found to be significantly higher than the other anti-cancer drugs as graphically represented in Figure 3. Also, cells not having Apoptotic protease activating factor 1, (Apaf-1) are also sensitive to Staurosporine (Str)-induced Apoptosis (Stepczynska et al., 2001).
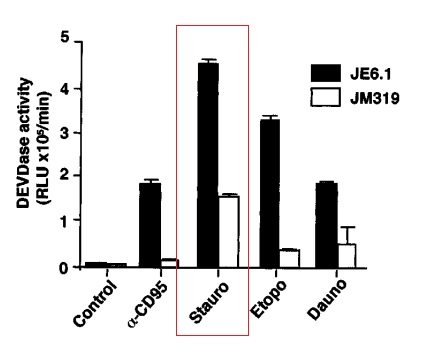
Figure 3 The highest Caspase Activity is induced by Staurosporine (Str, Stauro in this graph) in the JE6.1 and JM319 cells as highlighted by a red rectangle. The Caspase Activity induced by all the other anti-cancer drugs is relatively and significantly low as these cells are resistant to all the traditionally used anti-cancer drugs.
In a research study, Staurosporine (Str) has found to cause the induction of Apoptosis by the action of the activated caspase-9 (which belong to proteases class), in the cancer cells of pancreas, especially, PaTu 8988t and Panc-1 cells. The activated caspase-9 activates the protein working downstream of it, such as caspase-3 and caspase-6. Also in these cells, downregulated expression of two proteins, namely, B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated death promoter (BAD) were found.
Various research studies have been conducted to explore and understand the mechanism of Staurosporine (Str)-induced Apoptosis, but it is still an unresolved enigma. In one such study, the role of SAPK/JNK1 signalling pathway is found to be involved. In another study, the central role of Caspase-3 has been highlighted, which is normally present in the cells in inactive state, but becomes active as it receives apoptotic signals. These signals can be either radiations (ionizing in nature) or any pharmaceutically active ingredient, like Staurosporine (Str). Moreover, Staurosporine (Str)-induced Apoptosis is considered as a standard model to study the apoptosis in the Mammalian cells.
Along with the knowledge of mechanism of Apoptosis, another critical factor for the development of an effective anticancer drug is to limit the non-specific behaviour of Staurosporine (Str), so that its pharmacological action can be controlled and even made targeted only for one kinase protein. To attain this specificity, numerous derivatives from the basic structure of Staurosporine (Str) has been synthesized (Toledo and Lydon, 1997). For example: 3-Hydroxystaurosporine, UCN-01, Tetrahydrostaurosporine, K252a, KT5720, SB-218078. Some of them have reached the clinical trial phase of research; these are, Midostaurin (PKC412), Lestaurtinib (CEP-701), CP-751, CEP-1347, Edotecarin and Becatecarin. Moreover, the Staurosporine (Str) analogues which inhibit the kinases, are classified based on the differences at the certain positions in the original Staurosporine (Str) structure. The common positions include the residue sugar, the peripheral ring, and indolocarbazole ring (Gani and Engh, 2010).
Cell-impermeable staurosporine (CIMSS) is another analogue of Staurosporine (Str), with the same broad scope of inhibitory activity. It is therefore, the most suitable compound to study the extracellular kinases-dependent processes. It essentially prevents the Herpes simplex virus (HSV)-induced phosphorylation of PKB (thus, PKB remains active as explained earlier), followed by blocking the entry of Herpes simplex virus (HSV) in the cells. CIMSS also prevents the 3-phosphoinositide dependent protein kinase 1 (PDPK1) and phospholipase C gamma (PLCγ) from getting phosphorylated outside of the cell. Both, PDPK1 and PLCγ are produced in the Herpes simplex virus (HSV) infection. Moreover, CIMSS inhibits the SARS-CoV-2 infection.
CIMSS has been used in different studies to study the location of phosphorylation, in terms of being either intracellular or extracellular. Along with that, it has also been employed to study the mechanism of getting entered in the host cell for the HSV and SARS-CoV-2. Also, CIMSS has been employed in different research studies to investigate those viruses which use the kinase-dependent signalling pathway to help the entry of virus in the cell. In these studies, specifically, VSV pseudotyped with SARS-CoV-2 spike is found to be potentially inhibited by CIMSS. By using CIMSS, it has been uncovered that the SARS-CoV-2 spike protein has the potential to activate outside-in PLSCR1-Akt signalling pathway. In a research study, CIMSS is recognized as a novel anticancer drug with the potential benefits of having no activity with the non target molecules/ cells because it is impermeable to the cell, i.e., it cannot pass across the cell membrane (Malsy et al., 2019).
References:
GANI, O. A. & ENGH, R. A. 2010. Protein kinase inhibition of clinically important staurosporine analogues. Natural product reports, 27, 489-498.
MALSY, M., BITZINGER, D., GRAF, B. & BUNDSCHERER, A. 2019. Staurosporine induces apoptosis in pancreatic carcinoma cells PaTu 8988t and Panc-1 via the intrinsic signaling pathway. Eur J Med Res, 24, 5.
OMURA, S., SASAKI, Y., IWAI, Y. & TAKESHIMA, H. 1995. Staurosporine, a potentially important gift from a microorganism. J Antibiot (Tokyo), 48, 535-48.
STEPCZYNSKA, A., LAUBER, K., ENGELS, I. H., JANSSEN, O., KABELITZ, D., WESSELBORG, S. & SCHULZE-OSTHOFF, K. 2001. Staurosporine and conventional anticancer drugs induce overlapping, yet distinct pathways of apoptosis and caspase activation. Oncogene, 20, 1193-202.
TOLEDO, L. M. & LYDON, N. B. 1997. Structures of staurosporine bound to CDK2 and cAPK – new tools for structure-based design of protein kinase inhibitors. Structure, 5, 1551-1556.













Comments