The development process and application of mRNA engineering
Introduction to Nucleic Acid Research
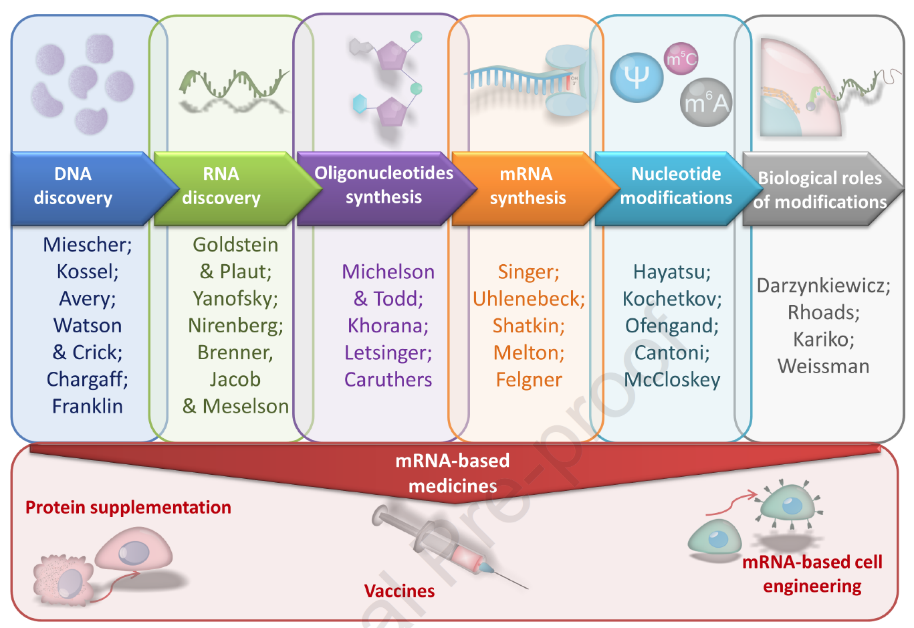
DNA Discovery
The first two centuries were marked by the elucidation of the structure and function of nucleic acids. Friedrich Michel was the first to isolate nuclear proteins in 1869. The name nucleic acid is a coined term that Albrecht Kossel divided into five bases in the late 19th century: adenine, cytosine, guanine, thymine and uracil. Another half century later, Ossivard Avery discovered that deoxyribonucleic acid (DNA) was involved in heredity, laying the foundation for molecular biology genetics. In addition, Chargaff's biochemical discovery of fixed ratios of certain bases, as well as Franklin's crystallographic studies, led Watson and Crick to discover the structure and function of DNA in 1953. However, the role of RNA and its connection to DNA and proteins remain unclear.
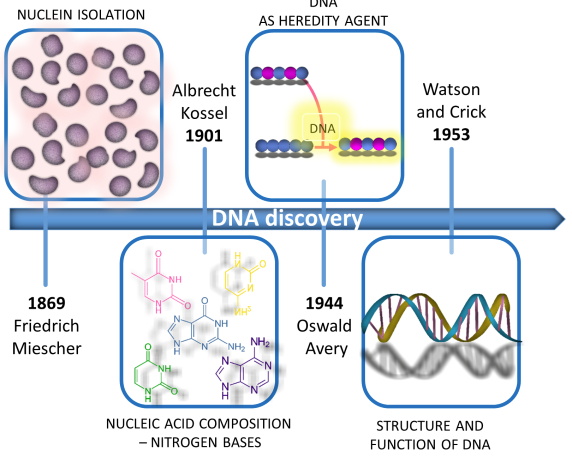
mRNA Discovery
Goldstein and Prout observed nuclear transfer in amoeba, and the subsequent identification of ribosomes and transfer RNAs, indicated where RNA synthesis was located in the mid-1950s. It wasn't until the early 1960s that extensive work demonstrated a third type of RNA—the soluble but short-lived messenger RNA (mRNA). The results in the two Nature articles published at the time proved that mRNA was a completely new class of RNA. At the same time, other RNA molecules of similar size and attached to ribosomes were also demonstrated. An article on DNA and transient RNA complementarity further addresses the difficult problem of coding transfer from DNA to protein. In 1964, Charles Yanofsky reported the collinearity of gene structure and protein structure. Nirenberg elucidated the genetic code and its role in protein synthesis in 1966, and was awarded the Nobel Prize two years later. In this way, the era of mRNA exploration began.
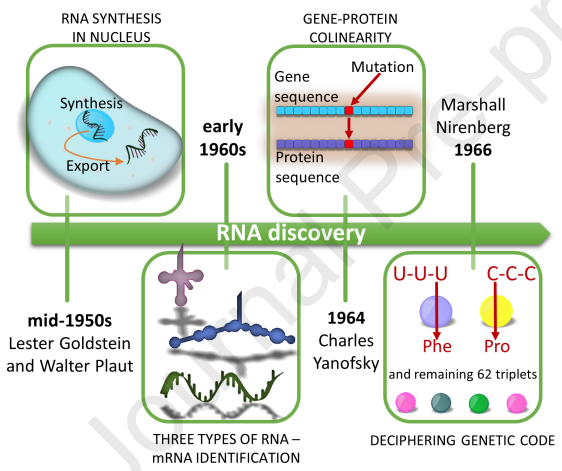
Oligonucleotide Synthesis
The discovery that nucleic acids are the source of genetic information quickly spurred the scientific research that led to the creation of synthetic codes, beginning with the chemical synthesis of dinucleotides by Michelson and Todd in 1955. Longer oligonucleotides can be synthesized by a new phosphodiester method introduced in the early 1960s by Korana et al. involving protection of the 2' and 5'-hydroxyl groups in ribonucleosides and subsequent condensation acid chain. Oligodeoxyribonucleotides have become the focus of research due to their stability and their role in the storage of genetic information. Synthetic oligonucleotides are essential for cracking the genetic code. This method is sufficient for the synthesis of oligodeoxyribonucleotides, which can then be added to double-stranded DNA as a template for the first biologically meaningful RNA molecule: alanine transfers ribonucleic acid in 1970. However, the lack of phosphate protection leads to branching of the oligonucleotide chain, and changing this requires laborious purification tasks at each step, making it impractical to attempt to synthesize longer oligonucleotides. The phosphate triester solid-state method introduced by Lessinger in the 1960s and 1970s addressed the shortcomings of the Korana method. This method, which served as the basis for the first oligonucleotide synthesizer, is simple enough and can be replicated quickly. However, stepwise efficiency and long coupling times complicate extending nucleotide chains beyond 20 bases. Carruthers fine-tuned by replacing the troublesome multimer with an inorganic approach and replacing a chlorine group with an amine, which is necessary to facilitate large-scale nucleic acid production because phosphoramidites can be prepared in advance, stored, and Easily activate before use.
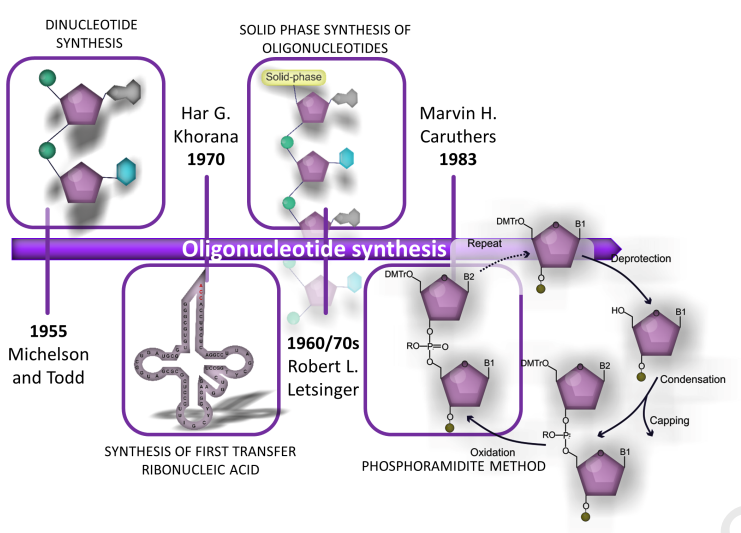
mRNA Production
The use of polynucleotide phosphorylases and ribonuclease A to facilitate the synthesis of oligoribonucleotides was achieved as early as the 1950s and further developed in the 1960s. The chemical synthesis of oligoribonucleotides from nucleoside 2'-O-benzyl ethers offers another option. The application of ribonuclease T1 in 1969 allowed the enzymatic synthesis of oligoribonucleotides with defined base sequences. A DNA-dependent RNA polymerase isolation method was reported in the same year. Then, in 1973, the in vitro production of short ribonucleotides based on DNA templates was demonstrated. Stepwise enzymatic oligoribonucleotide synthesis, including modified nucleotides, was reported two years later. However, none of these ribonucleotides could be used for protein production until the discovery of key in vitro protein synthesis.
At the same time, the results of the research on the structure of mRNA, combined with the results of the above-mentioned synthetic developments, made possible the production of mRNA. A single-stranded, adenine-rich RNA was found to correspond to the poly-A tail of mRNA in a study of reoviruses. It was subsequently determined that translation of eukaryotic mRNA requires a 5'-terminal 7-methylguanosine cap, and the translation initiation region of eukaryotic mRNA was characterized. In 1983, the first in vitro synthesis of functional mRNA using the SP6 phage promoter as a template and SP6 polymerase was fused to a human gene. Nearly a decade later, mRNA-based direct in vivo gene transfer into mouse muscle was reported. However, research has been hampered by the short duration of protein production and the potential to trigger an immune response.
Nucleotide and Nucleoside Modifications
First, scientists conducted preliminary studies on nucleoside modifications with the aim of finding therapeutic targets for alkylating agents. A follow-up study in 1964 revealed the selective modification of cytidine residues in RNA by semicarbazide. A large number of similar studies followed. However, most of these research efforts are directed at rRNA. Therefore, studies on artificial modifications are conducted in parallel with naturally occurring RNA modifications, which are most commonly found in tRNA, rRNA, mRNA, snRNA, and other small RNAs. Interestingly, tRNA nucleotide modifications contribute to the codon-dependent rate of nucleotide polymerization, another source of biological variability and a mechanism for regulating cellular function, providing opportunities for mRNA codon optimization to accelerate protein production.
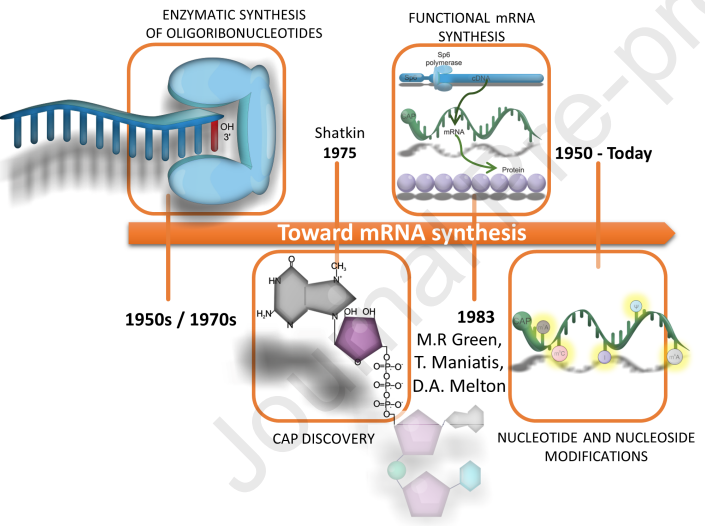
Translational Biology of Nucleotide Modifications
Nucleotide and nucleoside modifications facilitate the widespread use of mRNAs as therapeutics. They also constitute the most important page in the history of mRNA development. Two of the key points to address are: translational efficacy and immunogenicity. Pioneering work in these areas has contributed most to the great success of mRNA, including a highly potent COVID-19 vaccine. The efficacy of mRNA translation is critical for a number of reasons. Higher transformation efficacy requires lower mRNA load, thereby helping to reduce immunogenicity. Flanking mRNA regions are the most critical regulators of translational efficacy. As early as 1981, methyl esterification of m7G5'p was shown to reversibly block its activity as a eukaryotic mRNA 5'-cap analog. However, during in vitro transcription, up to half of the traditional m7GpppG caps are incorporated into the mRNA in the opposite orientation and cause loss of function. In 2001, the anti-reverse cap analog (ARCA) was introduced as an antidote, a new analog that overcomes the above limitations and always binds in the correct orientation. ARCA has been used extensively over the past two decades and has shown excellent results. Initially, scientists thought that ARCA just doubled translation efficiency, but a study on lipofection of dendritic cells showed that it increased translation efficiency by a factor of 20 and extended with Poly(A) tails from 64 to 100 glands. The synergistic effect of glycosides further increasing the efficiency to 35-fold, resulting in a total of 700-fold increase in the yield of the final reporter gene. Thus, ARCA exists as the design inspiration and basis for a series of new cap analogs over the ensuing two decades. A phosphorothioate cap analog based on the ARCA concept has been patented and exclusively acquired by BioNTech (which designs vaccines against COVID-19) for its research. Only recently did TriLink synthesize Cap 1 mRNA with co-transcribed CleanCap® analogs, which are more efficient than enzymatic reactions, which is especially important when large amounts of mRNA need to be produced in a short period of time. Thus, four decades of research into cap analogs have resulted in mRNA molecules with excellent efficiency and stability, making them suitable for a wide range of therapeutic uses. It is also the driving force behind the advancement of science.
In addition to translational efficiency issues, the immunogenicity of mRNA quickly dampened enthusiasm for the rapid translation of mRNA-based medicines into clinical practice. However, Dr. Kariko's findings demonstrate that the incorporation of pseudouridine into mRNA prevents immediate immune responses in the form of systemic interferon-alpha (IFN151 alpha) spikes following intravenous administration. In addition, pseudouridine also enhances translation by less significantly activating RNA-dependent protein kinase (PKR), which then phosphorylates translation initiation factor 2-alpha (eIF-2α) and inhibits translation of uridine-containing transcripts. Nucleoside modifications in RNA also limit 2'-5'-oligoadenylate synthase activation and increase resistance to ribonuclease L cleavage in another cellular sensing system. Modifications in the biological process of pseudouridine production can further eliminate impurities thereby attenuating the immune-activating properties of therapeutic mRNAs. Incorporation of pseudouridine and ARCA significantly increased yield, improved translation of modRNA and decreased its in vitro immunogenicity. Therefore, decorating crude mRNA with modified nucleotides can improve the compatibility of the organism. At present, the research on nucleotide modification has reached a certain level, and mRNA has also become a drug suitable for many fields including vaccines, protein supplementation and stem cell engineering, which is very promising.
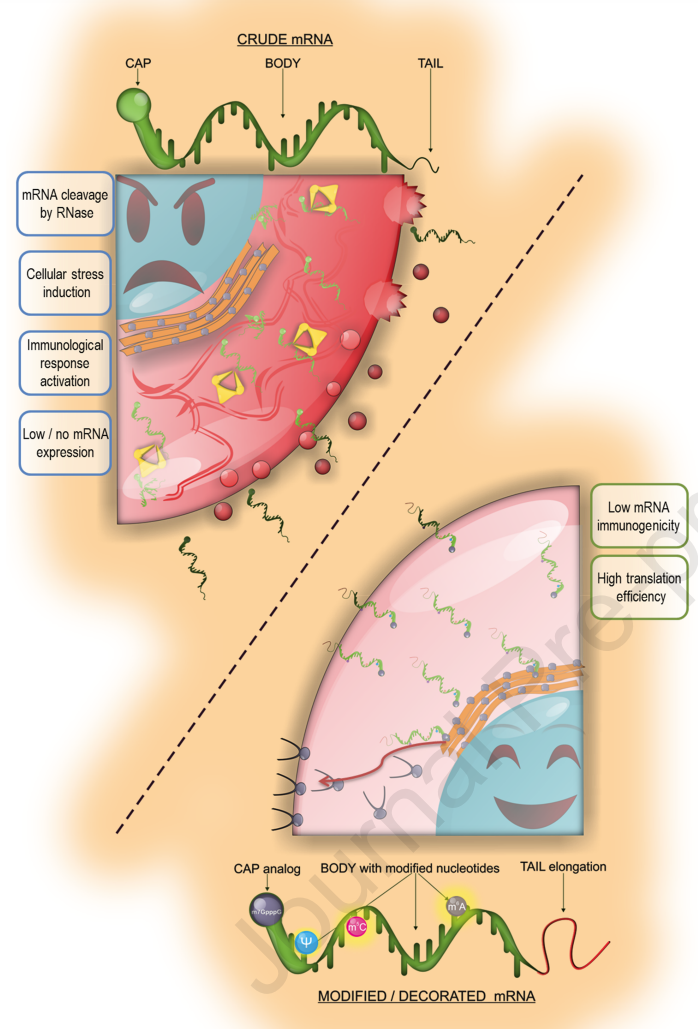
The Biological role of mRNA-based drugs
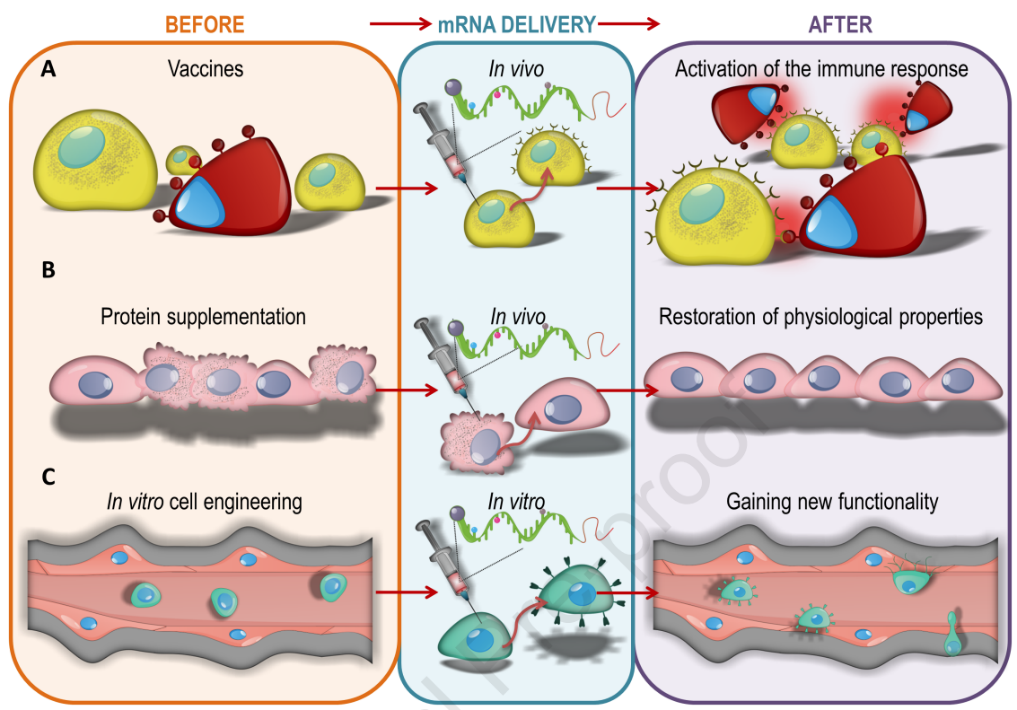
a) Vaccines
Vaccines are an indispensable tool in public health and occupy an important place in medicine. However, because vaccines are typically used in large numbers of healthy people, their efficacy and safety requirements are very strict. Earlier methods using attenuated or inactivated microorganisms were very effective, but safety issues were controversial, and production, purification and quality control were cumbersome and expensive. Protein-based vaccines, although less disadvantageous, require immune adjuvants and may awaken dormant autoimmunity. In addition, DNA-based vaccines are plagued by barriers to entry into cells and the nucleus and the need for additional equipment to support this process. The problem has recently been addressed by packaging DNA into harmless viruses, but it remains controversial. Either method almost requires living or structurally intact organisms for production, making development slower and more expensive. In this context, the concept of using mRNA appears to be the holy grail in vaccine development. mRNA vaccines can be obtained within days by sequencing microbial genetic material and simply mimicking the nucleotide sequences of receptor-binding domains (RBDs), then synthesizing them in robotic factories. Consequently, the time span from microbial discovery to clinical-grade vaccine candidates is extremely short. The simplicity of the overall process also allows for rapid response and easy adjustment of primary vaccines to account for microbial genetic drift and variant formation. In addition, there is no risk of contamination from foreign materials, which reassures the public. Taken together, these features seem to favor mRNA as an ideal vaccine solution.
b) Protein supplementation
Since proteins are key molecules in the intracellular and extracellular parts of all organisms, their loss or abnormality often leads to disease. Protein supplementation is an effective treatment. While there are multiple approaches to delivering proteins, such as viral-based gene replacement or direct protein delivery, there is also growing interest in using mRNA to achieve this goal. Unlike DNA, mRNA is not integrated with the genome, which avoids many safety concerns. Physically all mRNA molecules are relatively similar, so the same formulation may serve many genes. At the same time, the huge variation in protein properties has led to the need for individual development of the formulation and delivery of each protein. The preparation of mRNA for protein supplementation is more demanding than vaccines. While induction of a host immune response is welcome in vaccine applications, it is paramount to prevent the immunogenicity of mRNA delivered for protein supplementation, especially since protein supplementation may be administered throughout the lifespan and immunogenic material may lead to chronic inflammation.
c) mRNA-based cell engineering
Stem cell and immune cell therapy are emerging as important areas of medical research, and cell engineering is often used to increase therapeutic potential. A variety of cellular functions can be achieved by administering mRNA. At the same time, this technique is crucial for transient cell modification. mRNA-based cell engineering can well address cell trafficking and migration issues. Studies have shown that the adhesion molecule integrin α4 (ITGA4) can be expressed on the surface of mesenchymal stem cells (MSCs) after encoding mRNA. MSC migration was also increased in vitro by mRNA encoding CXCR4. The same strategy can also enhance the homing of natural killer cells. The expression of active immune molecules is another large area of mRNA-based cell engineering. The delivery of chimeric antigen receptor (CAR) or T cell receptor (TCR) mRNA to lymphocytes produces potent immune cells. While its short-lived nature can be overcome with multiple infusions, short-term expression can prevent severe complications due to immune cells attacking themselves. mRNA-based technologies are also well suited for genome editing. The Cas9 enzyme can also be delivered to cells as mRNA along with an associated guide RNA (gRNA) sequence. mRNA is also a viable strategy to induce pluripotency in somatic cells.










Comments