BODIPY 505/515
|
| Catalog No.GC42960 |
BODIPY 505/515, a lipophilic fluorescence dye, emits fluorescence has been used extensively for lipid droplet labeling (Ex/Em: 505/515 nm).
Products are for research use only. Not for human use. We do not sell to patients.
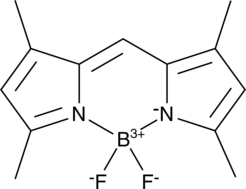
Cas No.: 21658-70-8
Sample solution is provided at 25 µL, 10mM.
BODIPY 505/515, a lipophilic fluorescence dye, emits fluorescence has been used extensively for lipid droplet labeling (Ex/Em: 505/515 nm) [1,2] . BODIPY 505/515 is compatible with epifluorescent, confocal, and two-photon microscopy, and flow cytometry, and can be used for live and fixed cell applications. Bodipy 505/515 is used for labeling a broad variety of lipids, e.g., phospholipids, fatty acids, cholesteryl esters, cholesterol, and ceramides
References:
[1]. Velmurugan, N., Sathishkumar, Y., Yim, S.S., et al. Study of cellular development and intracellular lipid bodies accumulation in the thraustochytrid Aurantiochytrium sp. KRS101. Bioresour. Technol. 161, 149-154 (2014).
[2]. Rumin, J., Bonnefond, H., Saint-Jean, B., et al. The use of fluorescent Nile red and BODIPY for lipid measurement in microalgae. Biotechnol. Biofuels 8, 42 (2015).
[3]. Elle I C, Olsen L C B, Pultz D, et al. Something worth dyeing for: molecular tools for the dissection of lipid metabolism in Caenorhabditis elegans[J]. FEBS letters, 2010, 584(11): 2183-2193.
Average Rating: 5 (Based on Reviews and 10 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *





