BODIPY-Cholesterol (Synonyms: BCh2) |
| Catalog No.GC42964 |
BODIPY-Cholesterol is cholesterol tagged with a boron dipyrromethene difluoride (BODIPY) fluorophore used for monitoring sterol uptake and inter-organelle sterol flux in cells with excitation of 480 nm and emission of 508 nm.
Products are for research use only. Not for human use. We do not sell to patients.
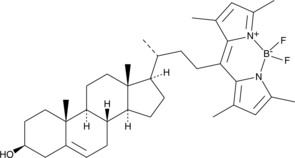
Cas No.: 878557-19-8
Sample solution is provided at 25 µL, 10mM.
BODIPY-Cholesterol is cholesterol tagged with a boron dipyrromethene difluoride (BODIPY) fluorophore used for monitoring sterol uptake and inter-organelle sterol flux in cells with excitation of 480 nm and emission of 508 nm.[1] The excretion of BODIPY cholesterol from late endoplasmic organelles depends on acidic lipase and Niemann pickc1 protein.[6]
In vitro, treatment with Bodipy-cholesterol in cells, prominent PM labeling is observed at 2–5 min; however, upon ≥30 min incubations, it is also observed the fluorescence labeling of intracellular structures.[2] In vitro efficacy test it suggested that accumulation of BODIPY-cholesterol in the media gave more reproducible values than assaying for the loss of the compound from the cells.[3] With 1 μg BODIPY-cholesterol analogs show a similar cellular localization in HeLa cells and exhibit similar cholesterol efflux properties from THP1 cells to HDL acceptors.[4] BODIPY-cholesterol efflux clearly increased when treatment of fibroblasts with the Hh pathway agonist SAG, which enhances Ptc protein expression, or over-expression of human Ptc in yeast.[5] Treatment with miR-758 inhibitor obviously increased ABCA1-dependent cholesterol efflux by BODIPY-Cholesterol efflux assay.[7] Micrographs of EPCs incubated with HDL labeld bodipy-cholesterol (50 µg/ml) 30 min, small cytoplasmic vesicles as well as large positive MVBs containing intraluminal microvesicles, tightly-packed with reaction products could be displayed. The internalized-HDL-derived bodipy-cholesterol was also spread within many of the stacked Golgi cisterns and the TGN.[8]
References:
[1].Wüstner D, et al. Potential of BODIPY-cholesterol for analysis of cholesterol transport and diffusion in living cells. Chem Phys Lipids. 2016 Jan;194:12-28.
[2].Hölttä-Vuori M, et al. BODIPY-cholesterol: a new tool to visualize sterol trafficking in living cells and organisms. Traffic. 2008 Nov;9(11):1839-49.
[3].Sankaranarayanan S, et al. A sensitive assay for ABCA1-mediated cholesterol efflux using BODIPY-cholesterol. J Lipid Res. 2011 Dec;52(12):2332-2340.
[4].Liu Z, et al. Synthesis of cholesterol analogues bearing BODIPY fluorophores by Suzuki or Liebeskind-Srogl cross-coupling and evaluation of their potential for visualization of cholesterol pools. Chembiochem. 2014 Sep 22;15(14):2087-96.
[5].Bidet M, et al. The hedgehog receptor patched is involved in cholesterol transport. PLoS One. 2011;6(9):e23834.
[6].Kanerva K, et al. LDL cholesterol recycles to the plasma membrane via a Rab8a-Myosin5b-actin-dependent membrane transport route. Dev Cell. 2013 Nov 11;27(3):249-62.
[7].Yao Y, et al. Glucagon-like peptide-1 contributes to increases ABCA1 expression by downregulating miR-758 to regulate cholesterol homeostasis. Biochem Biophys Res Commun. 2018 Mar 4;497(2):652-658.
[8].Srisen K, et al. Human endothelial progenitor cells internalize high-density lipoprotein. PLoS One. 2013 Dec 30;8(12):e83189.
Average Rating: 5 (Based on Reviews and 16 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




