Cadazolid (Synonyms: ACT-179811) |
| Catalog No.GC19085 |
Cadazolid (ACT-179811) is a new oxazolidinone antibiotic with potent activity against Clostridium difficile.
Products are for research use only. Not for human use. We do not sell to patients.
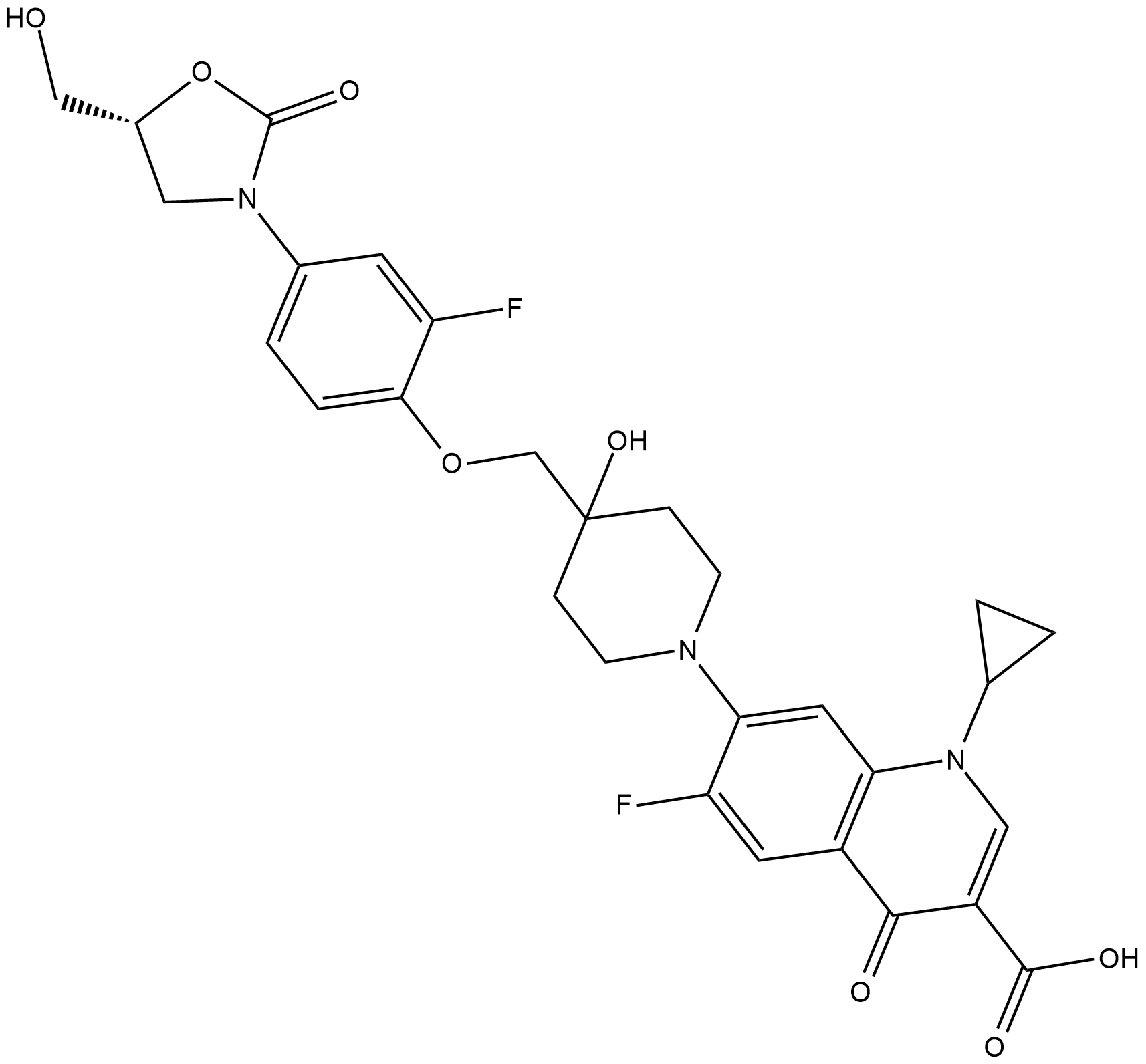
Cas No.: 1025097-10-2
Sample solution is provided at 25 µL, 10mM.
Cadazolid (ACT-179811) is a new oxazolidinone antibiotic with potent activity against Clostridium difficile.
Cadazolid is a new antibiotic in development for the treatment of Clostridium difficile-associated diarrhea[1]. Cadazolid is active against all (including linezolid- and moxifloxacin-resistant) Clostridium difficile strains (MIC90 0.125, range 0.03-0.25 mg/L). The cadazolid geometric mean MIC is 152-fold, 16-fold, 9-fold and 7-fold lower than those of moxifloxacin, linezolid, metronidazole and vancomycin, respectively. Both cadazolid dosing regimens rapidly reduce Clostridium difficile viable counts and cytotoxin with no evidence of recurrence. Cadazolid levels persists at 50-100-fold supra-MIC for 14 days post-dosing. Cadazolid inhibition of enumerated gut microflora is limited, with the exception of bifidobacteria; Bacteroides fragilis group and Lactobacillus spp. counts are unaffected. There is no evidence for selection of strains resistant to cadazolid, quinolones or linezolid[2].
Cadazolid is well tolerated up to 3000 mg given twice daily for 10 days. The most common adverse event is headache, with no observed relationship between dose or treatment duration and adverse events. Plasma concentrations of cadazolid are low. No plasma concentrations >3.3 ng/mL are observed after single doses or >6.9 ng/mL after 10 days of multiple doses. Food increased the mean Cmax from 0.73 to 1.87 ng/mL and mean AUC0-t from 3.13 to 15.69 ng.h/mL after a single 300 mg dose. The increase in systemic exposure to cadazolid across doses is less than dose-proportional. The mean cumulative faecal recovery is 81.0%-93.5%. Urinary recovery of unchanged compound is less than 0.015%[1].
References:
[1]. Baldoni D, et al. Cadazolid, a novel antibiotic with potent activity against Clostridium difficile: safety, tolerability and pharmacokinetics in healthy subjects following single and multiple oral doses. J Antimicrob Chemother. 2014 Mar;69(3):706-14.
[2]. Chilton CH, et al. In vitro activity of cadazolid against clinically relevant Clostridium difficile isolates and in an in vitro gut model of C. difficile infection. J Antimicrob Chemother. 2014 Mar;69(3):697-705.
Average Rating: 5 (Based on Reviews and 9 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















