Ulipristal |
| Catalog No.GC17305 |
progestogen receptor modulator
Products are for research use only. Not for human use. We do not sell to patients.
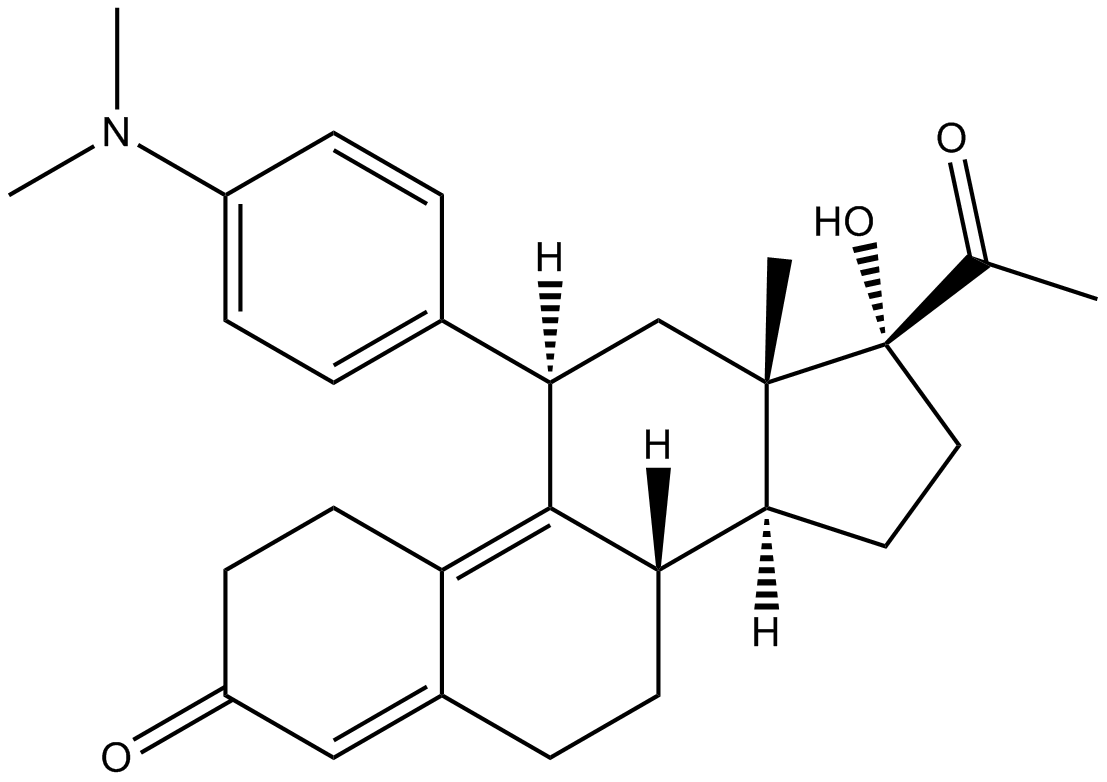
Cas No.: 159811-51-5
Sample solution is provided at 25 µL, 10mM.
Ulipristal is a novel and selective progesterone receptor modulator (SPRM) for emergency contraception after an unprotected intercourse or contraceptive failure. Ulipristal can be used for the treatment of benign gynecological conditions such as uterine myoma [1]. SPRMs is a new class of agent which can act on the progesterone receptor. SPRMs have been involved in exerting clinically relevant tissue-selective progesterone agonist, antagonist, or mixed agonist/antagonist effects on progesterone target tissues [2].
In vitro: Ulipristal acetate dose dependently suppressed progesterone-induced acrosome reaction and hyperactivation in human spermatozoa. Ulipristal acetate modulated human sperm functions by acting as progesterone antagonists [3].
In vivo: In the terminal deoxynucleotide transferase–mediated dUTP nick-end labeling assay, UPA-treated myomas increased cell death when compared with untreated myomas [4].
Clinical trials: UPA seemed to be a reasonably tolerable and effective method of emergency contraception (EC)when used within 120 hours of intercourse. UPA is at least as effective as LNG when used in the first 72 hours after unprotected intercourse [1].
References:
[1]. Richardson A R, Maltz F N. Ulipristal acetate: review of the efficacy and safety of a newly approved agent for emergency contraception[J]. Clinical therapeutics, 2012, 34(1): 24-36.
[2]. Chwalisz K, Perez M C, DeManno D, et al. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis[J]. Endocrine reviews, 2005, 26(3): 423-438.
[3]. Ko J K Y, Huang V W, Li R H W, et al. An in vitro study of the effect of mifepristone and ulipristal acetate on human sperm functions[J]. Andrology, 2014, 2(6): 868-874.
[4]. Courtoy G E, Donnez J, Marbaix E, et al. In vivo mechanisms of uterine myoma volume reduction with ulipristal acetate treatment[J]. Fertility and sterility, 2015, 104(2): 426-434. e1.
Average Rating: 5 (Based on Reviews and 38 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















