Amiloride HCl (Synonyms: MK-870) |
| Catalog No.GC17853 |
Amiloride HCl (MK-870 hydrochloride) is an inhibitor of both epithelial sodium channel (ENaC[1]) and urokinase-type plasminogen activator receptor (uTPA[2]).
Products are for research use only. Not for human use. We do not sell to patients.
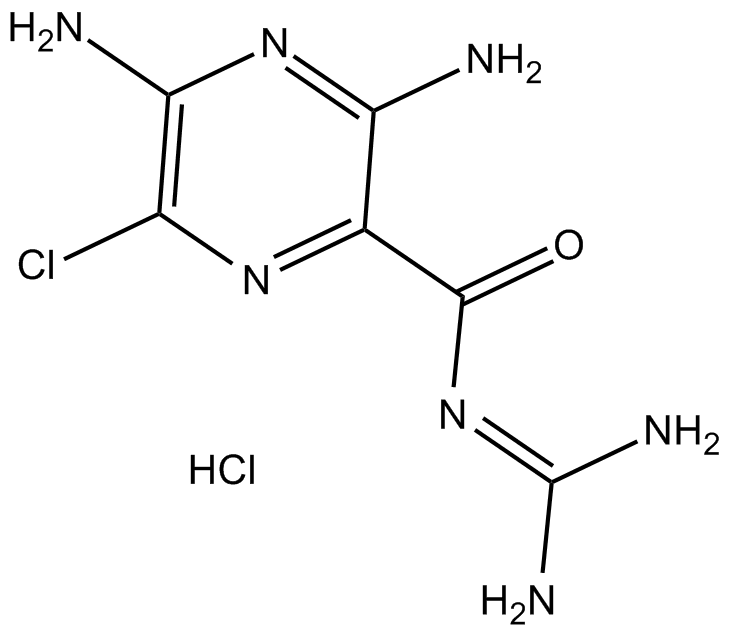
Cas No.: 2016-88-8
Sample solution is provided at 25 µL, 10mM.
The epithelial sodium channel (ENaC) is a membrane-bound ion-channel that is permeable to protons, Li+-ions, and especially Na+-ions. It is a constitutively active ion-channel. It can be argued that it is the most selective ion channel. In vertebrates, the channels control reabsorption of sodium in kidney, colon, lung and sweat glands. They also play a role in taste perception. Amiloride is a relatively selective inhibitor of the epithelial sodium channel (ENaC).
In vitro: Amiloride is a relatively poor inhibitor of the the Na+/H+ exchanger (NHE) with an IC50 of 3 μM in the presence of a low external [Na+] but as high as 1 mM in the presence of a high [Na+]. Amiloride completely inhibits Na+ influx in doses of 1.5 μM in vascular smooth muscle cells [1].
In vivo: Amiloride at 1 mg/kg/day is found to reverse the initial increases in collagen deposition and prevent further increases in the DOCA-salt hypertensive rat. Amiloride delays the onset of proteinuria and improved brain and kidney histologic scores in the stroke-prone spontaneously hypertensive rats. Amiloride antagonizes or prevents actions of aldosterone in cardiovascular and renal tissues in animals with salt-dependent forms of hypertension [1].
Clinical trial: Amiloride is a potassium-sparing diuretic, first approved for use in 1967, used in the management of hypertension and congestive heart failure. Amiloride was also tested as treatment of cystic fibrosis, but it was revealed inefficient in vivo due to its short time of action.
Reference:
[1] Teiwes J, Toto RD. Epithelial sodium channel inhibition in cardiovascular disease. A potential role for amiloride. Am J Hypertens. 2007 Jan;20(1):109-17.
Average Rating: 5 (Based on Reviews and 36 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















