2-NBDG
|
| Catalog No.GC10289 |
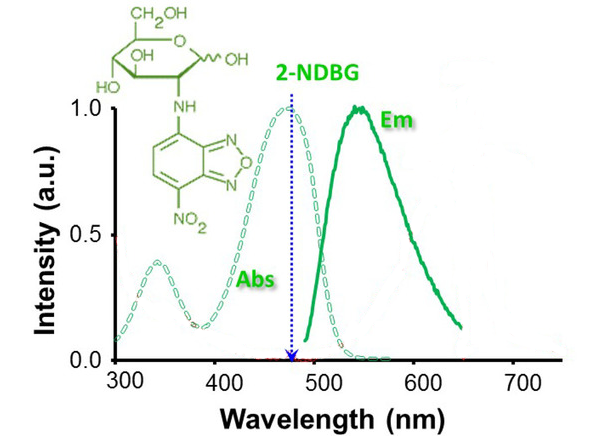
2-NBDG is a fluorescence-labeled 2-deoxy-glucose analog useful as a tracer for evaluation of cellular glucose metabolism (Ex/Em: 475/550 nm).
Products are for research use only. Not for human use. We do not sell to patients.
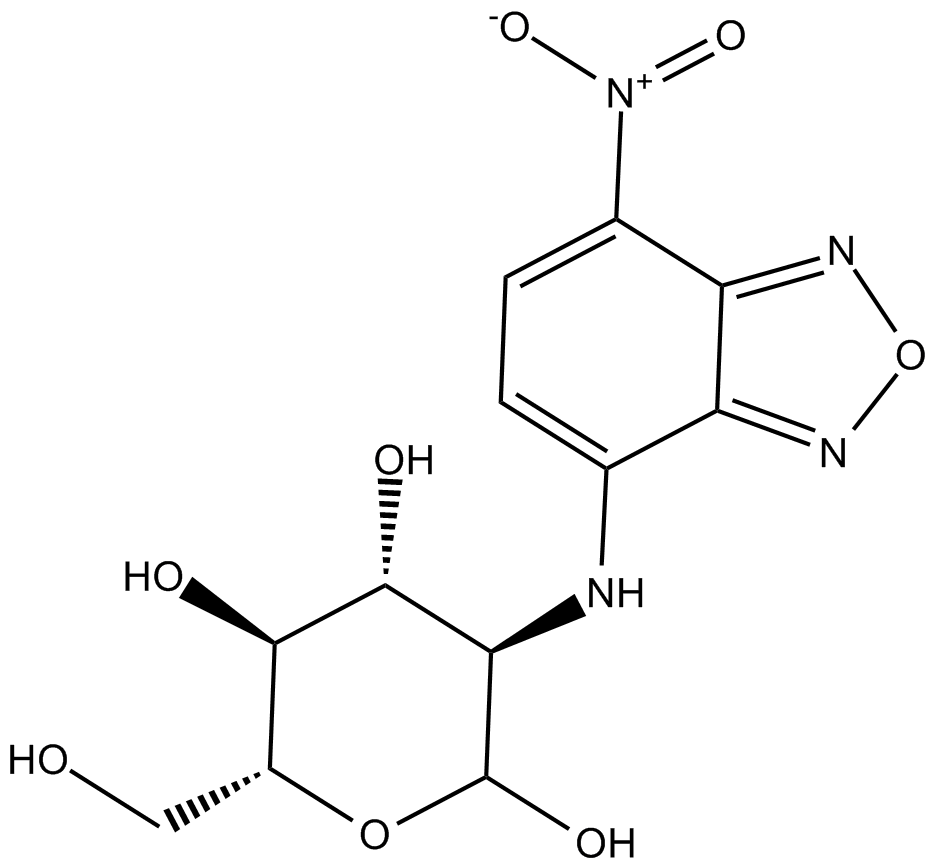
Cas No.: 186689-07-6
Sample solution is provided at 25 µL, 10mM.
- Ecotox Environ Safe 243 (2022): 113996.PMID:36030680
- Bioorganic Chemistry (2023): 106341.
- Oncogene (2023): 1-11.
- J Cardiovasc Dev Dis (2023): 10(6) 246.
- Front Pharmacol 13 (2022): 875014.PMID:35694255
- Ind Eng Chem Res (2023).
- Phytomedicine 114 (2023): 154740.PMID:36965373
- Nat Commun 15.1 (2024):557.PMID:38228638
- Tradit Med Res 9.7 (2024):37.
- Endocrinol Diab Meta 7.3 (2024).PMID:38556697
2-NBDG is a fluorescence-labeled 2-deoxy-glucose analog useful as a tracer for evaluation of cellular glucose metabolism (Ex/Em: 475/550 nm).
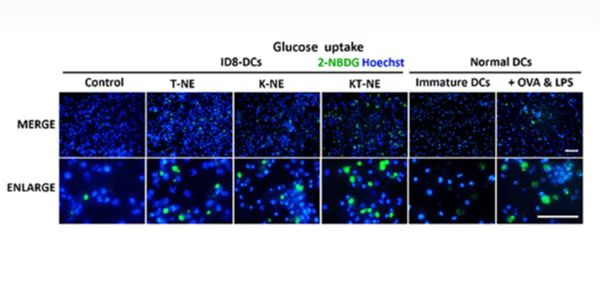
Glucose is a necessary source of energy for sustaining cell activities and homeostasis in tissues. Glucose metabolism is an important target in many diseases and changed with the pathological condition, therefore, evaluation of glucose metabolism can be a significant indication in disease progressions.
2-NBDG can be used in many kinds of cells in vitro, such as HepG2 human hepatocarcinoma cells, L6 rat skeletal muscle cells, MCF-7 breast cancer epithelial cells and astrocytes, it is also used in disease models, epilepsy rat, hyperglycemia, diabetes or mouse xenograft model of cancer.
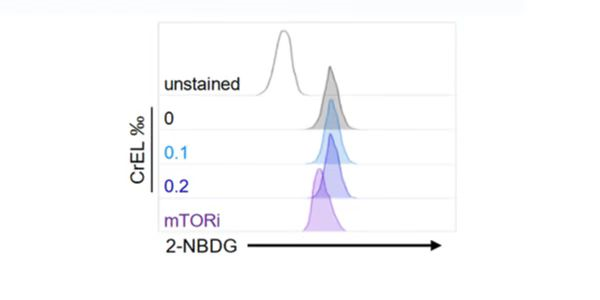
2-NBDG enters cells through glucose transporters and is subsequently phosphorylated by hexokinase and trapped inside cells. Flow cytometric detection of fluorescence produced by cells can be performed to examine 2-NBDG uptake into living cells, and the intracellular concentration of transported 2-NBDG can be measured with a fluorescence microplate assay. It can be detected with a fluorescence imaging microscopy or CCD camera simply as well.
2-NBDG is a fluorescently labeled glucose tracer that is transported into cells by the same glucose transporter (GLUT) as glucose. Once 2-NBDG is taken up by cells, it is phosphorylated at the C-6 position to give 2-NBDG-6-phosphate, which is well retained in the cell. Compared to other glucose tracers such as 2-DG or FDG, 2-NBDG enables in situ measurement of 2-NBDG with high temporal and spatial resolution at the single-cell level. (suitable for fluorescence microscopy and flow cytometry detection)
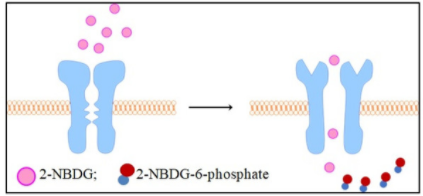
Rationale for 2-NBDG glucose uptake assay in cells: Once 2 NBDG is taken up by cells, it is phosphorylated at the C-6 position to generate 2-NBDG-6-phosphate in 2 NBDG metabolism, which is well retained in the cell, the fluorescence intensity is proportional to the cellular glucose uptake activity.
References:
[1]. Zou C, Wang Y, Shen Z. 2-NBDG as a fluorescent indicator for direct glucose uptake measurement[J]. Journal of biochemical and biophysical methods, 2005, 64(3): 207-215.
[2]. O’Neil R G, Wu L, Mullani N. Uptake of a fluorescent deoxyglucose analog (2-NBDG) in tumor cells[J]. Molecular Imaging and Biology, 2005, 7(6): 388-392.
[3]. Tsytsarev V, Maslov K I, Yao J, et al. In vivo imaging of epileptic activity using 2-NBDG, a fluorescent deoxyglucose analog[J]. Journal of neuroscience methods, 2012, 203(1): 136-140.
[4]. Yan Chen, Junjian Zhang, Xiang-yang Zhang, 2-NBDG as a Marker for Detecting Glucose Uptake in Reactive Astrocytes Exposed to Oxygen-Glucose Deprivation In Vitro. J Mol Neurosci (2015) 55:126–130.
[5]. Tsytsarev V, Maslov K I, Yao J, et al. In vivo imaging of epileptic activity using 2-NBDG, a fluorescent deoxyglucose analog[J]. Journal of neuroscience methods, 2012, 203(1): 136-140.
Average Rating: 5 (Based on Reviews and 39 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *