Cefotaxime (sodium salt) (Synonyms: CTX) |
| Catalog No.GC16544 |
Cefotaxime (Cefotaxim) sodium, a β-lactamase stable cephalosporin and a third-generation cephalosporin antibiotic, possesses broad-spectrum antibiotic activity against numerous Gram-positive and Gram-negative bacteria.
Products are for research use only. Not for human use. We do not sell to patients.
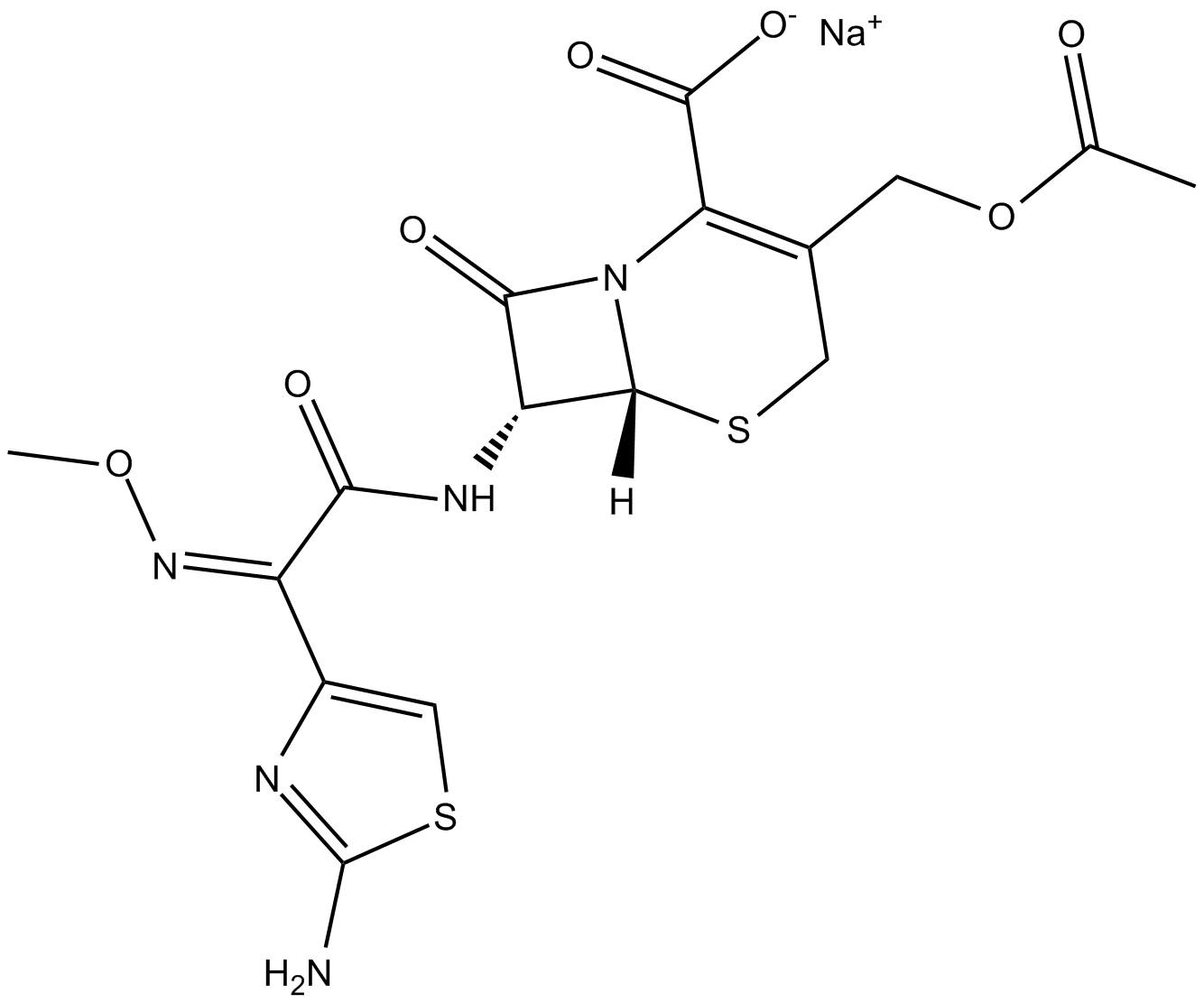
Cas No.: 64485-93-4
Sample solution is provided at 25 µL, 10mM.
MIC: <0.1 μg/ml for S. pneumoniae
Cefotaxime is a cephalosporin antibiotic.
The cephalosporins, a class of β-lactam antibiotics originally derived from the fungus Acremonium, are indicated for the prophylaxis and treatment of infections caused by bacteria susceptible to this particular form of antibiotic.
In vitro: Previous studies found that the in-vitro activity of cefotaxime against Staphylococcus au reus has ranged from 0.8 g to 8 μg/ml with 50% of isolates inhibited by 2 μg/ml and 90% by 4 μg/ml. Moreover, it was found that methicillin resistant S. aureus were resistant to cefotaxime with MIC values above 64 μg/ml. The cefotaxime MICs against S. pneumoniae were found to be below 0.1 μg/ml, with 90% inhibited by 0.04 μg/ml. Cefotaxime has also been shown to have excellent activity against Haemophilus injluenzae, such as β-lactamase-containing strains [1].
In vivo: An in-vivo study with the mouse model of Vibrio vulnificus infection was conducted to evaluate the efficacies of therapy with minocycline or cefotaxime alone and in combination. Results indicated that combination therapy with cefotaxime and minocycline was distinctly more advantageous than therapy with the single antibiotic regimen for the treatment of severe Vibrio vulnificus infections [2].
Clinical trial: A clinical trial was conducted to test the efficacy and safety of cefotaxime in the therapy of community-and hospital-acquired pneumonias. The results suggested that cefotaxime would be an affective and well-tolerated antimicrobial agent for the treatment of pneumonia becuause of susceptible organisms [3].
References:
[1] Neu HC. The in vitro activity, human pharmacology, and clinical effectiveness of new beta-lactam antibiotics. Annu Rev Pharmacol Toxicol. 1982;22:599-642.
[2] Chuang YC, Ko WC, Wang ST, Liu JW, Kuo CF, Wu JJ, Huang KY. Minocycline and cefotaxime in the treatment of experimental murine Vibrio vulnificus infection. Antimicrob Agents Chemother. 1998 Jun;42(6):1319-22.
[3] Schleupner CJ, Engle JC. Clinical evaluation of cefotaxime for therapy of lower respiratory tract infections. Antimicrob Agents Chemother. 1982 Feb;21(2):327-33.
Average Rating: 5 (Based on Reviews and 36 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















