Daclatasvir (Synonyms: BMS 790052) |
| Catalog No.GC40756 |
Daclatasvir is a first generation direct-acting inhibitor of hepatitis C virus (HCV) non-structural protein 5A (NS5A; Kds = 8 and 210 nM for the NS5A33-202 and NS5A26-202 residues of HCV genotype 1b, respectively).
Products are for research use only. Not for human use. We do not sell to patients.
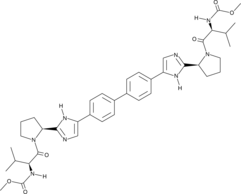
Cas No.: 1009119-64-5
Sample solution is provided at 25 µL, 10mM.
Daclatasvir is a potent HCV NS5A protein inhibitor, with mean EC50 values of 50 and 9 pM against genotype 1a and 1b replicons, respectively.
Daclatasvir (BMS-790052) is a small molecule inhibitor of the HCV NS5A protein that exhibits picomolar half-maximum effective concentrations (EC50) towards replicons expressing a broad range of HCV genotypes and the JFH-1 genotype 2a infectious virus in cell culture. Daclatasvir is a potent inhibitor of the JFH-1 genotype 2a infectious virus that replicates in cell culture (EC50=28 pM), an assay considered to be a more biologically relevant in vitro cell culture system. In addition, Daclatasvir displays similar potency in Huh-7, HeLa and HEK293T cells, demonstrating that the function(s) of NS5A inhibited by Daclatasvir is (are) highly conserved in different cellular environments[1].
In a randomized, double-blind, placebo-controlled, single ascending-dose study, Daclatasvir (BMS-790052) is administered at six dose levels to healthy, non-HCV-infected subjects over a range of 1 to 200 mg as an oral solution. Daclatasvir is safe and well tolerated up to 200 mg with no clinically relevant adverse effects. After oral administration, Daclatasvir is readily absorbed, with dose-proportional exposures over the studied dose range, and all subjects have drug concentrations greater than the protein-binding-adjusted EC90 for genotypes 1a and 1b, as measured in the replicon assay, at and beyond 24 h post-dose. (The protein binding-adjusted EC90 figures are derived from an analysis of the effect of the addition of human serum on antiviral activity in replicons. In the presence of 40% human serum, the EC90 for Daclatasvir is 383 pM (0.28 ng/mL) for the genotype 1a replicon and 49 pM (0.04 ng/mL) for the genotyope 1b replicon)[1]. Mice in each group that developed persistent HCV infection are divided into two treatment groups. One group receive 4 weeks of Asunaprevir/Daclatasvir treatment and the other group received 4 weeks of Ledipasvir/GS-558093 treatment. Asunaprevir/Daclatasvir therapy and Ledipasvir/GS-558093 therapy rapidly decease serum HCV RNA levels to below the sensitivity, and they are not detected after completion of the therapy except for two mice in the Ledipasvir/GS-558093 group[2].
References:
[1]. Gao M, et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature. 2010 May 6;465(7294):96-100.
[2]. Kai Y, et al. Emergence of hepatitis C virus NS5A L31V plus Y93H variant upon treatment failure of daclatasvir and asunaprevir is relatively resistant to ledipasvir and NS5B polymerase nucleotide inhibitor GS-558093 in human hepatocyte chimeric mice. J Ga
[3]. Zhang X, et al. Discovery and evolution of aloperine derivatives as a new family of HCV inhibitors with novel mechanism. Eur J Med Chem. 2018 Jan 1;143:1053-1065.
Average Rating: 5 (Based on Reviews and 36 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















