α,β-Methyleneadenosine 5'-triphosphate (sodium salt) (Synonyms: αβ-methylene ATP) |
| Katalog-Nr.GC45195 |
α,β-Methyleneadenosine 5'-Triphosphat (Natriumsalz), ein PhosphonsÄureanalogon von ATP, ist ein P2X3- und P2X7-Rezeptorligand.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 1343364-54-4
Sample solution is provided at 25 µL, 10mM.
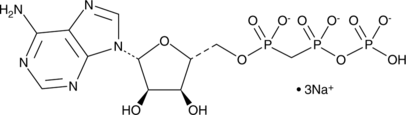
α,β-Methyleneadenosine 5’-triphosphate (αβ-methylene ATP) is a phosphonic analog of ATP that is characterized by the replacement of the bridging oxygen atom between the α- and β-phosphate groups with methylene. It is an agonist of P2X purinoceptors P2X1 and P2X3 (EC50 = ~1 µM) and is ~1,000-fold less potent at P2X2, P2X receptors 4-7, and P2Y receptors. [1][2][3][4] Persistent activation of purinoceptors results in desensitization, resulting in an antagonist-like effect of αβ-methylene ATP. [5] αβ-methylene ATP is used as a stable analog of ATP to study the interaction of ATP with kinases and other proteins. [6][7] It weakly binds and inhibits adenylate cyclase in a calcium-dependent manner (Ki= ~0.5 mM). [8][9]
Reference:
[1]. North, R.A. Molecular physiology of P2X receptors. Physiological Reviews 82(4), 1013-1067 (2002).
[2]. Coddou, C., Yan, Z., Obsil, T., et al. Activation and regulation of purinergic P2X receptor channels. Pharmacological Reviews 63(3), 641-683 (2011).
[3]. Helms, N., Kowalski, M., Illes, P., et al. Agonist antagonist interactions at the rapidly desensitizing P2X3 receptor. PLoS One 8(11), 1-11 (2013).
[4]. Abbracchio, M.P., Burnstock, G., Boeynaems, J.M., et al. International Union of Pharmacology LVIII: Update on the P2Y G protein-coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacological Reviews 58(3), 281-341 (2006).
[5]. Dunn, P.M. Fertility: Purinergic receptors and the male contraceptive pill. Current Biology 10(8), R305-R307 (2000).
[6]. Gibson, K.J., Schubert, K.R., and Switzer, R.L. Binding of the substrates and the allosteric inhibitor adenosine 5'-diphosphate to phosphoribosylpyrophosphate synthetase from Salmonella typhimurium. The Journal of Biological Chemisty 257(5), 2391-2396 (1982).
[7]. Steegborn, C., Litvin, T.N., Levin, L.R., et al. Bicarbonate activation of adenylyl cyclase via promotion of catalytic active site closure and metal recruitment. Nature Structural & Molecular Biology 12(1), 32-37 (2005).
[8]. Krug, F., Parikh, I., Illiano, G., et al. αβ-Methylene-adenosine 5'-triphosphate. A competitive inhibitor of adenylate cyclase in fat and liver cell membranes. The Journal of Biological Chemisty 248(4), 1203-1206 (1973).
[9]. Dessauer, C.W., Scully, T.T., and Gilman, A.G. Interactions of forskolin and ATP with the cytosolic domains of mammalian adenylyl cyclase. The Journal of Biological Chemisty 272(35), 22272-22277 (1997).
Average Rating: 5 (Based on Reviews and 17 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















