Almonertinib mesylate (Synonyms: HS-10296 mesylate) |
| Katalog-Nr.GC64398 |
Almonertinib (HS-10296)-Mesylat ist ein oral verfÜgbarer, irreversibler EGFR-Tyrosinkinase-Inhibitor der dritten Generation mit hoher SelektivitÄt fÜr EGFR-sensibilisierende und T790M-Resistenzmutationen. Almonertinib-Mesylat zeigt eine starke inhibitorische AktivitÄt gegen T790M, T790M/L858R und T790M/Del19 (IC50: 0,37, 0,29 bzw. 0,21 nM) und ist weniger wirksam gegen den Wildtyp (3,39 nM). Almonertinib-Mesylat wird zur Erforschung des nicht-kleinzelligen Lungenkrebses eingesetzt.
Products are for research use only. Not for human use. We do not sell to patients.
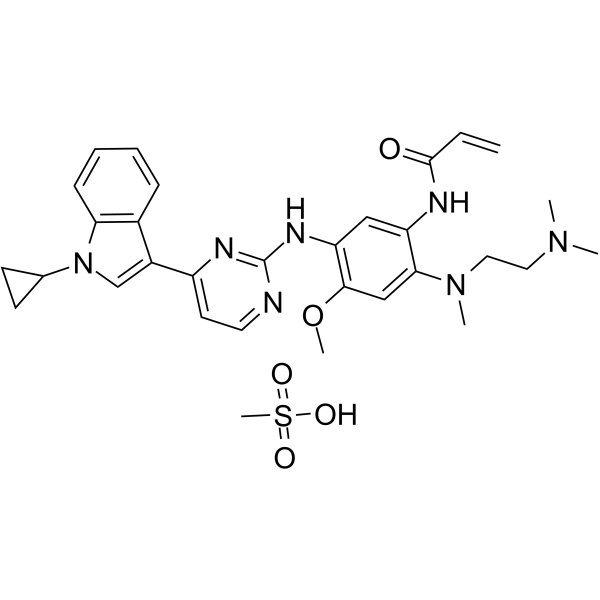
Cas No.: 2134096-06-1
Sample solution is provided at 25 µL, 10mM.
Almonertinib (HS-10296) mesylate is an orally available, irreversible, third-generation EGFR tyrosine kinase inhibitor with high selectivity for EGFR-sensitizing and T790M resistance mutations. Almonertinib mesylate shows great inhibitory activity against T790M, T790M/L858R and T790M/Del19 (IC50: 0.37, 0.29 and 0.21 nM, respectively), and is less effective against wild type (3.39 nM). Almonertinib mesylate is used for the research of the non-small cell lung cancer[1][2].
HS-10296 mesylate is an orally available inhibitor of the epidermal growth factor receptor (EGFR) mutant form T790M, with potential antineoplastic activity, which canbe used to treat NSCLC[2]. Additionaly, HS-10296 mesylate could also inhibit other EGFR sensitive mutations, including G719X, del19, L858R and L861Q[3].
[1]. Yang JC, et al. Safety, Efficacy, and Pharmacokinetics of Almonertinib (HS-10296) in Pretreated Patients With EGFR-Mutated Advanced NSCLC: A Multicenter, Open-label, Phase 1 Trial [published online ahead of print, 2020 Sep 9]. J Thorac Oncol. 2020;S1556-0
[2]. Sullivan I, et al. Next-Generation EGFR Tyrosine Kinase Inhibitors for Treating EGFR-Mutant Lung Cancer beyond First Line. Front Med (Lausanne). 2017 Jan 18;3:76.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















