3BDO Successfully Inhibits Glioblastoma
Growth in Vitro and in Vivo
Background
Glioblastoma (GBM) is a high-grade glioma characterized by a high rate of invasiveness and rapid growth. Despite advances in surgical and medical treatment modalities, very few patients with GBM survive beyond 15 months after diagnosis. Currently, temozolomide (TMZ) is the main first-line drug for GBM chemotherapy, but it is prone to drug resistance. Therefore, it is urgent to identify other drugs with better efficacy to treat GBM.
Cancer recurrence and metastasis are often attributed to cancer stem cells (CSCs), which have the ability to regenerate tumors by resisting standard chemotherapy and radiation regimens. Most cancers have been shown to contain CSCs, including GBM. CSCs in GBMs are termed glioma stem cells (GSCs) and have been shown to undergo multilineage differentiation, autonomous renewal and extensive proliferation and function as key molecules for GBM growth. Furthermore, epithelial-mesenchymal transition (EMT) is the mechanism by which normal epithelial cells acquire a mesenchymal phenotype. Cellular EMT is fundamental to cancer development, a process that increases the motility of individual cancer cells and allows them to invade epithelial junctions and the extracellular matrix. Multiple studies on GBM have demonstrated that activation of EMT-like programs enhances migration and invasion of malignant cells, and patients with aggressive GBM and poor prognosis consistently exhibit elevated levels of mesenchymal markers. Therefore, GSC and EMT inhibition may be a viable treatment for GBM.
Survivin is a protein related to the inhibition of apoptosis. More and more studies have found that compared with normal central nervous tissue, the expression level of survivin in glioma cells is significantly increased, and the expression of survivin is positively correlated with the grade of pathological glioma. Furthermore, elevated survivin expression promotes EMT and stemness processes. 3BDO is an mTOR activator that acts as an autophagy inhibitor. Studies have shown that 3BDO exposure can significantly reduce the number of autophagosomes in APP/PS1 transgenic mice and improve the memory function of the mice. Furthermore, 3BDO partially reversed the maturation of Rheb1-deficient neutrophils through mTOR inactivation. At the same time, there is increasing evidence that 3BDO can act independently as an mTOR regulator. For example, 3BDO attenuated plaque endothelial cell death and slowed the development of atherosclerosis in mice, but in a manner independent of autophagy or mTOR activity. 3BDO has also been shown to act as an inflammatory inhibitor both in vivo and in vitro. 3BDO has also been shown to inhibit the growth of cancer cells when combined with DPB. However, it is unclear whether 3BDO exerts potential anticancer properties in GBM.
Scientists from China have verified that 3BDO can successfully inhibit EMT and stemness processes in GBM through a series of in vitro and in vivo experiments. This study not only identifies 3BDO as a potential GBM drug, but also provides a rationale for a new approach to GBM treatment. The article was published in the Journal of Cancer.

Result
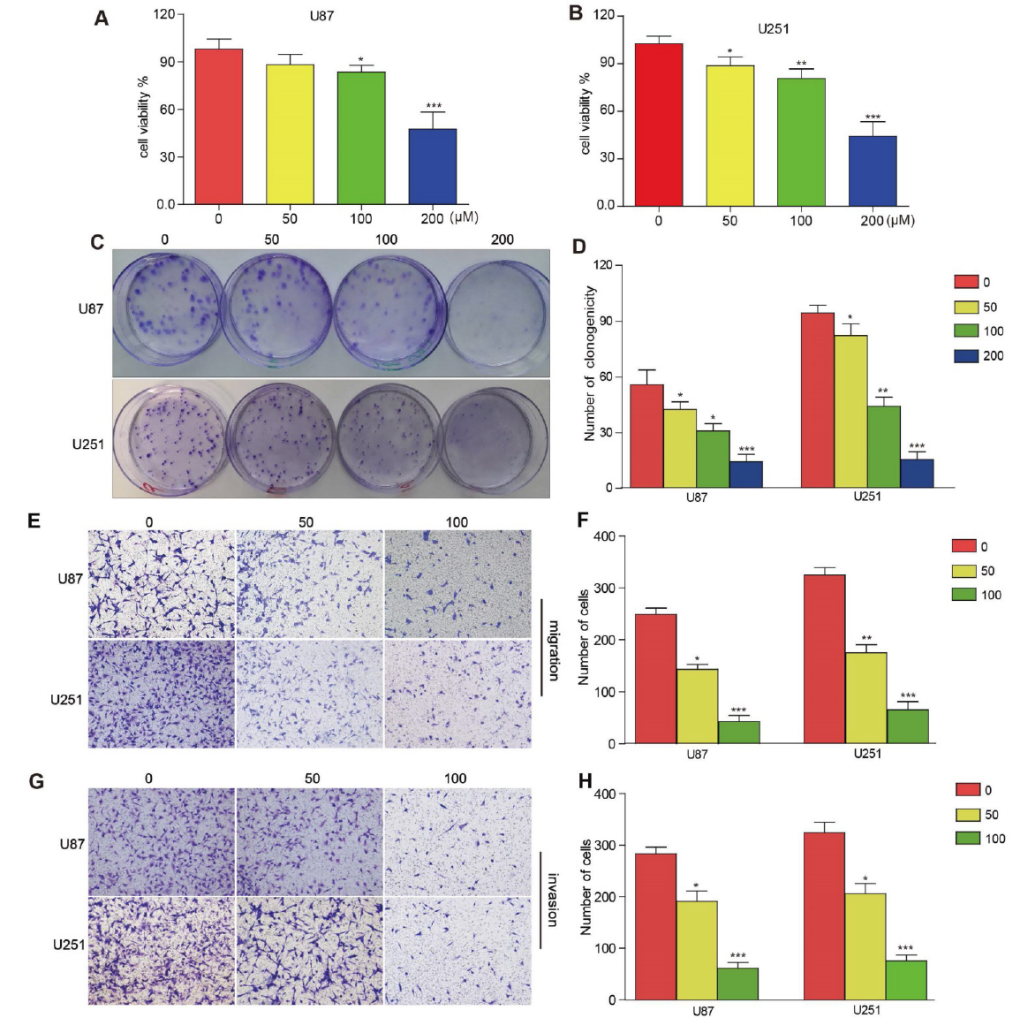
3BDO inhibits proliferation, migration and invasion of GBM cells
As shown in Figure 1 A-B, 3BDO inhibited the growth of U87 and U251 cells in a dose-dependent manner after 24 hours of treatment. Likewise, 3BDO also inhibited the colony-forming ability of the cells in a dose-dependent manner (Fig. 1C–D). The researchers then used transwell invasion assays to observe the invasive and migratory abilities of cells treated with 3BDO. The results showed that the 3BDO-treated group exhibited lower cell invasion and migration rates in a dose-dependent manner compared with the untreated group (Fig. 1E–H). These in vitro findings strongly support the ability of 3BDO to inhibit GBM cell proliferation, invasion and migration.
3BDO Reduces the Expression of EMT Markers and Inhibits the Expression of Survivin in GBM Cells
Exposure to 3BDO at doses of 50 μM or 100 μM for 24 hours resulted in lower expression levels of neural cadherin, vimentin and snail proteins than the 0 μM group (Fig. 1 I-M). Meanwhile, 3BDO decreased the expression level of survivin in a dose-dependent manner in U87 and U251 cells.
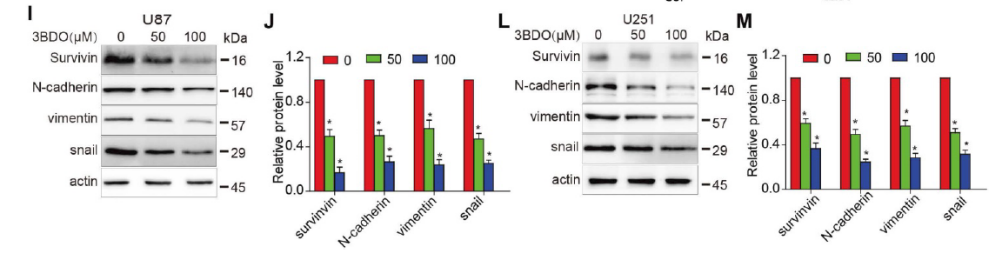
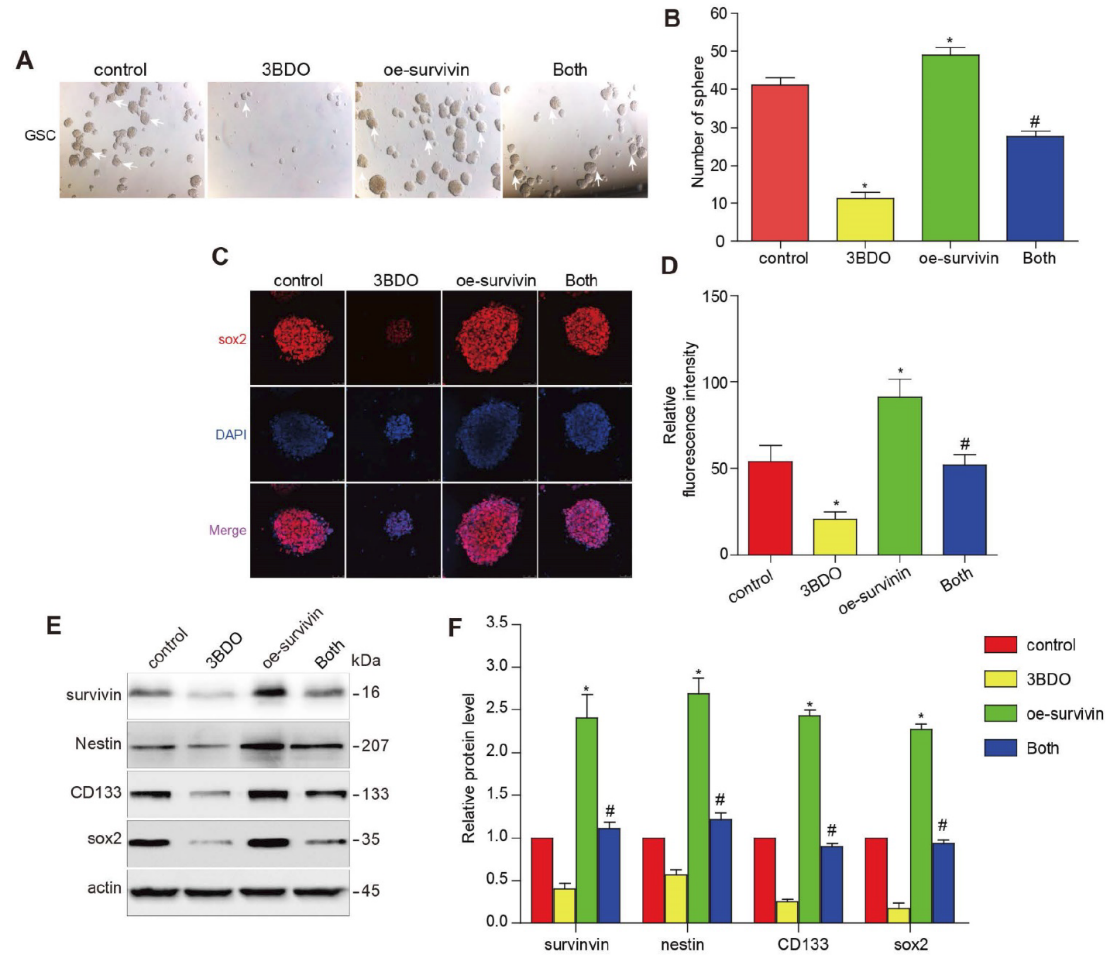
3BDO Inhibits Cell Growth and Downregulates the Expression of Survivin and Stemness Markers in GSCs
The occurrence and development of GBM is attributed to the action of GSC. Therefore, there is a sound scientific basis for GSCs as a therapeutic target for GBM. The researchers first cultured GSCs in pelleting medium and treated them with different concentrations of 3BDO for 72 hours. As shown in Figure 2A-B, 3BDO treatment reduced the number of GSC spheroids in a dose-dependent manner. Furthermore, sox2 is an important marker of GSC stemness. The researchers found that the expression level of sox2 decreased with the increase of 3BDO concentration (Fig. 2C-D). Next, they quantified the expression of stemness markers, such as nestin, CD133 and sox2, by western blot analysis. The results showed that the expression levels of the above-mentioned stemness markers were down-regulated after incubation with the indicated doses of 3BDO for 24 hours. Furthermore, 3BDO decreased the expression level of survivin in GSCs in a dose-dependent manner (Fig. 2E–F).
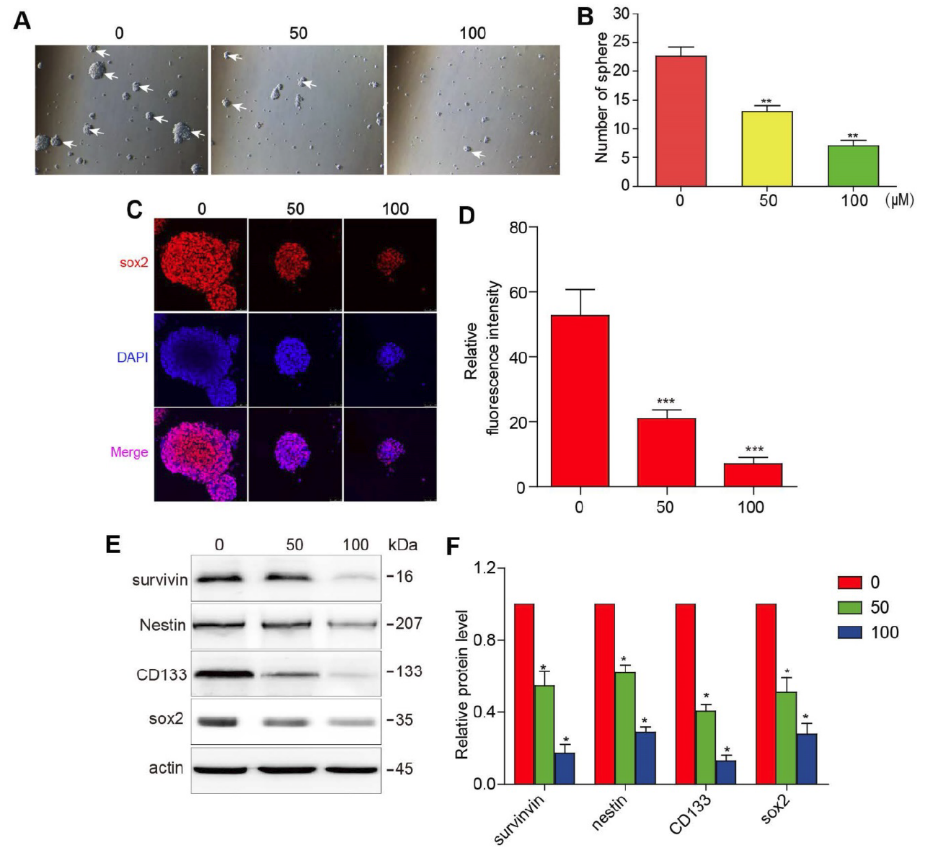
Overexpression of Survivin Reduces the Effect of 3BDO on GBM Cells and GSCs
U87 and U251 cells were artificially induced and overexpressed survivin prior to incubation in 3BDO or vehicle for 24 hours. In the presence of survivin overexpression, the proliferation, migration, and invasion abilities of GBM cells were increased (Fig. 3A-F). At the same time, upregulation of survivin increased the expression of EMT markers, including neural cadherin, vimentin, and snail proteins (Fig. 3 G-J). In addition, overexpression of survivin also reduced the inhibitory effect of 3BDO on GBM cells.
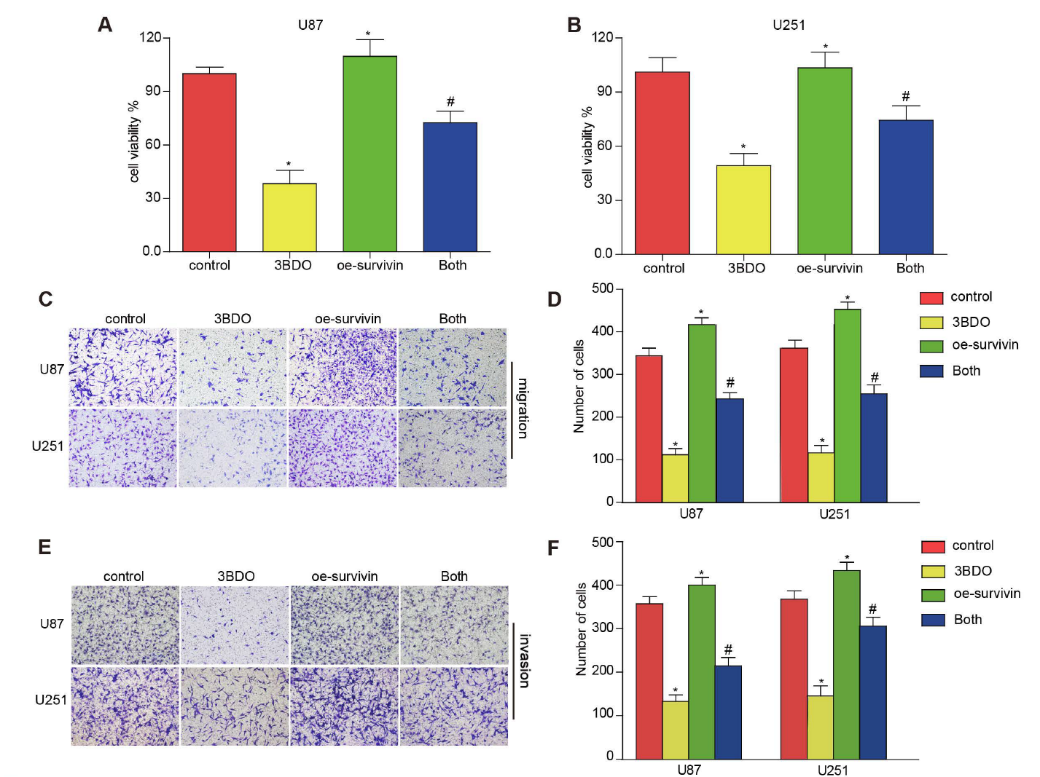
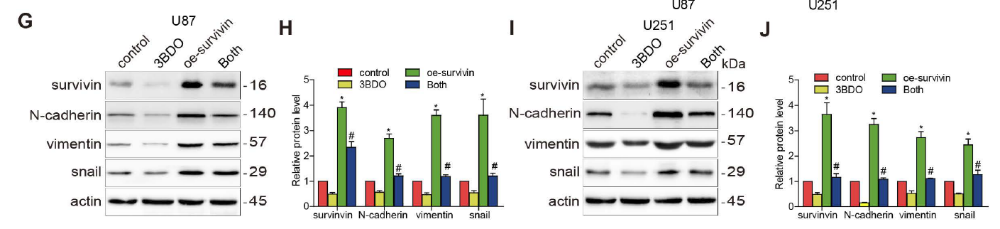
Multiple studies have reported that survivin is abundantly expressed in GBM tissues and human-derived GSC cultures. During GSC growth, both in vivo and in vitro downregulation of survivin was abolished. Whereas overexpression of survivin increased the number of spheroids formed and upregulated stemness markers such as nestin, CD133 and sox2. Furthermore, overexpression of survivin reduced the effect of 3BDO on spheroid formation and the upregulation of stemness markers in GSCs (Fig. 4). These results suggest that 3BDO exerts antitumor effects by inhibiting survivin-triggered EMT in GBM cells and by exerting anti-stem effects on GSCs.
3BDO Inhibits GBM Growth in U87 Xenograft Mouse Model
Compared with the untreated group, 3BDO treatment (80 mg/kg/day) over 25 days resulted in a significant reduction in tumor weight and volume (Fig. 5A-C) but did not affect mouse body weight (Fig. 5D). Furthermore, in tumors dissected from U87 xenograft mice, the expression of endogenous survivin, snail protein, cadherin, vimentin, and GSC markers such as nestin, sox2, and CD133 was inhibited by 3BDO (Figure 5 E-F). These findings are consistent with in vitro showing that 3BDO inhibits tumor growth by inhibiting survivin-mediated EMT and stemness processes in a glioblastoma xenograft mouse model, highlighting its potential as a therapeutic GBM candidate. In addition, in order to detect the liver and kidney toxicity of the drug, the researchers took the liver and kidney of the mice at the end of the experiment, sliced and stained them. The results showed that the liver and kidney of mice in the control group and 3BDO group were not damaged (Fig. 5G).
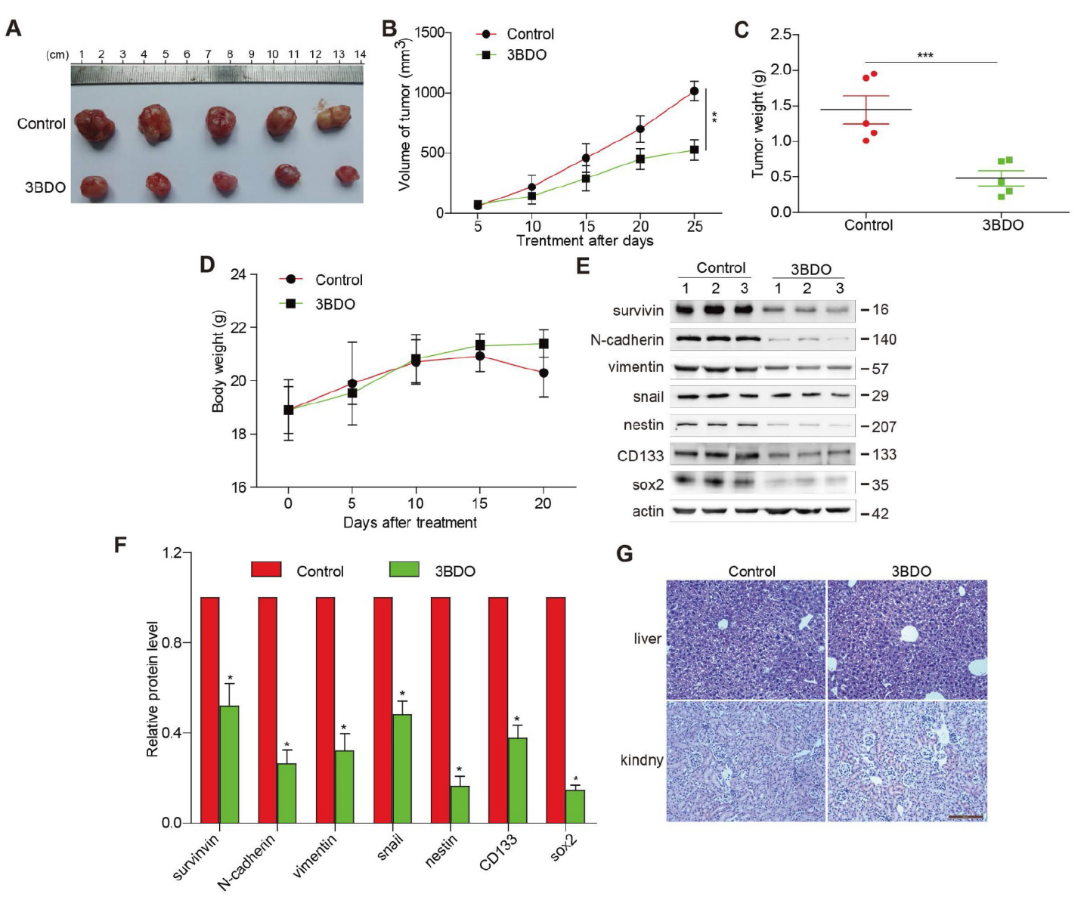
Overall, we demonstrate that 3BDO can successfully inhibit EMT and stemness processes in GBM both in vivo and in vitro, and identify survivin as a potential target of 3BDO. They also demonstrated for the first time that 3BDO exerts anti-GBM properties using a subcutaneous glioma model, which is consistent with its mechanism of action demonstrated in vitro. In addition, there were no significant changes in the total body weight of the mice and the histopathology of the liver and kidney, confirming that 3BDO was well tolerated. Therefore, 3BDO is a potential therapeutic drug for the treatment of GBM.













Kommentare
e
e