RSL3
RSL3 is also called RAS-selective lethal 3. It is a small molecule that can inhibit the action of GPX4, which leads to the ferroptosis of the cells. Ferroptosis is defined as the non-apoptotic type of cell death which is iron-dependent. In the presence of iron, or due to the accumulation of iron within the cells, various kinds of enzymes are active, which leads to ferroptosis. This increase in the accumulation of iron results in an increase in the level of lipid peroxidase enzymes within the cells. These enzymes upon lipid peroxidation produce reactive oxygen species. These ROS lead the cell to non-apoptotic cellular death. As we have discussed that glutathione peroxide 4 inhibits the cells to undergo ferroptosis. Although various small molecules are available that can activate the process of ferroptosis. RSL3 is one of them. It activates ferroptosis by inhibiting glutathione peroxide 4. One of the studies stated that RSL3 can be used for a therapeutic purpose to kill cancer cells through the process of ferroptosis. In this study, they demonstrate the effects of RSL3 on the three different types of colorectal cancer cells CRC (HT29, LoVo, and HCT116). In this research, they found that RSL3 can activate the process of non-apoptotic cell death, but it is time and dose-dependent. All this happens due to the increased level of ROS through an increase in the pool of iron in the cell. The study also stated that GPX4 inhibition plays a key role in ferroptosis induced by RSL3. An Overexpression of RSL3 on GPX4 protects the cells from ferroptosis. Hence RSL3 can be used as a cancer therapy for the treatment of colorectal cancer because of its ability to induce ferroptosis. It is because the colorectal cancer cells (CRC) on treatment with RSL3 lead to the increase in the expression of transferrin protein. This is accompanied by a decrease in the GPX4 expression, which is an indication of iron-dependent cell death.
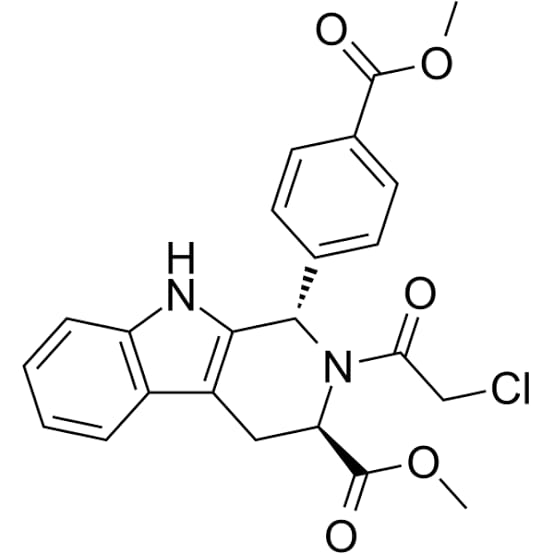
Figure 1: Structure of RSL3
Different studies also explained its application in the treatment of rhabdomyosarcoma, melanoma, renal cell carcinoma, and malignant glioma. In past, Treatment of Malignant glioma was a challenge because of the poor prognosis in the surgical excision along with radiotherapy and chemotherapy. So, a new therapy was urgently needed that can combat these issues. In past some research has been conducted which indicated that RSL3 can diminish Activating Transcription Factor 4 in various types of glioma cells I include, including U-251, and U-87. But no research was explained the therapeutic effect of RSL3 on malignant glioma. As we know that tumor cells shift their energy source from oxidative phosphorylation to glycolysis to fulfill their energy demand. In glioma cells, various enzymes like PKM2, HKII, and PFKP limit the process of glycolysis. This can contribute to the genesis and progression of tumors. An in-vitro study conducted on the effects of RSL3 on malignant glioma revealed that RSL3 can cause glycolysis dysfunction by decreasing the availability of the ATP, pyruvate, and enzymatic proteins PKM2, HKII, and PFKP. All this leads to the autophagy activation and autophagic death of glioma cells. This glycolysis dysfunction leads to the production of reactive oxygen species that in return leads to oxidative stress on the cancer cells. One of the mysterious facts which the researchers noticed during research is that supplementation of sodium pyruvate can decrease autophagy activation and autophagic cell death. It is because pyruvate molecules can maintain the level of glutathione peroxide 4 and 5 in the cells. This glutathione peroxide level decreases the production of reactive oxygen species in cells, hence preventing cellular death.
Similarly, glioblastoma which is the most aggressive form of malignant glioma also has a poor prognosis because of its high reoccurrence rate. RSL3 can also induce the process of ferroptosis by inhibiting glutathione peroxide 4 which increases the production of ROS and iron accumulation. Furthermore, studies also revealed that NF-κB also facilitates the ferroptosis induced by RSL3 in glioma cells. RSL3 in response, increase the production of reactive oxygen species. This will result in the activation of NF-κB. The activated NF-κB can downregulate the xCT and ATF4. Further, the activation of NF-κB also increases the release of inflammatory cytokines like IL-1β and tumor necrosis factor α. As we know that xCT and ATF4 give protection to the glioma cells from ferroptosis. Upon downregulation of ATF4, cells undergo ferroptosis. Thus, the RSL3 along with the chemotherapy approach can be a significant therapeutic tool for the treatment of glioblastoma .
On the same hand, RSL3 can also be used in the treatment regimen of prostate cancer because it can enhance the effects of cisplatin (DPP) as an anticancer. In past, cisplatin resistance is one of the major issues regarding the treatment of prostate cancer. Studies suggested that RSL3 along with the use of cisplatin can significantly reduce the viability of prostate cancer cells by inducing the process of ferroptosis. This ferroptosis is induced via inhibition of the GPX4 by RSL3 at low doses. Studies also indicated that RSL3 can enhance the sensitivity of prostate cancer cells to cisplatin. This RSL3 by improving the sensitivity increase the production of reactive oxygen species and increases the apoptotic rate of the cell. Furthermore, it decreases the availability of pyruvate, ATP, HKII, PKM2, etc. by interrupting the glycolysis process of PC cells. Interestingly in-vivo studies revealed, this cisplatin can also decrease the growth of prostate cancer cells with the least side effects. Thus, cisplatin chemotherapy along with the therapeutic use of RSL3 gives a good therapeutic approach for the treatment of prostatic cancer.
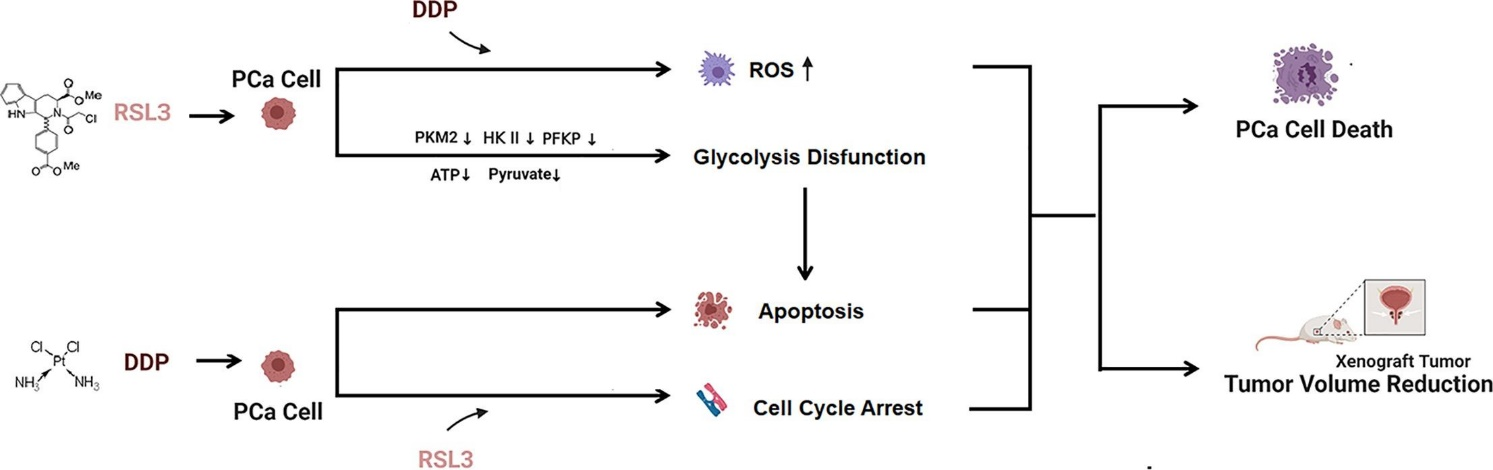
Figure 2: role of RSL3 and cisplatin (DDP) in prostatic cancer cell death.
Similarly, it ca also act as an inhibitor for lipopolysaccharide-induced inflammatory reactions. Studies exhibit that, upon stimulation of the RSL3, macrophages and microglia cells show resistance to ferroptosis by increasing the expression of Nrf2. This is how that Nrf2 inhibits the recruitment of the RNA Polymerase II and represses the process of cytokine transcription. This will protect the cells from ferroptosis. Although different types of macrophages and microglia cells exhibit a different level of sensitivity to RSL3-induced ferroptosis level.














Kommentare