Deoxynivalenol (Synonyms: 4-deoxy Nivalenol) |
| Catalog No.GC12969 |
Deoxynivalenol (DON) is a high-toxicity secondary metabolite produced by Fusarium graminearum.
Products are for research use only. Not for human use. We do not sell to patients.
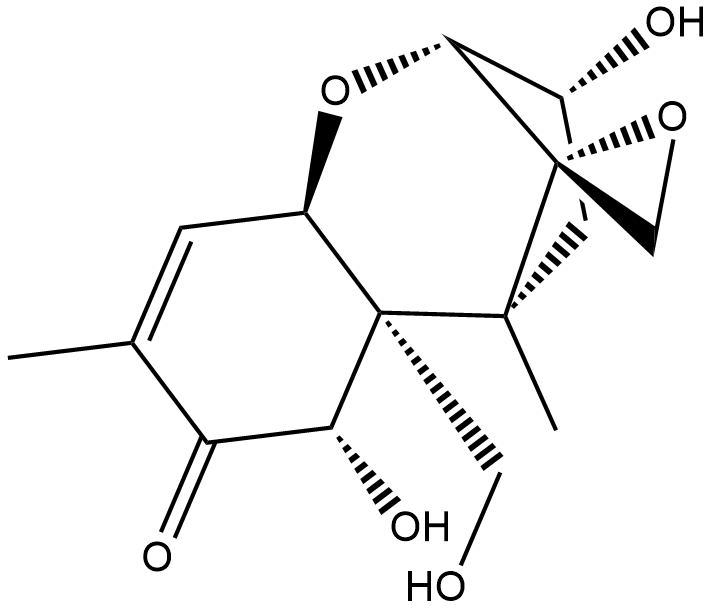
Cas No.: 51481-10-8
Sample solution is provided at 25 µL, 10mM.
Deoxynivalenol (DON) is a high-toxicity secondary metabolite produced by Fusarium graminearum. It is one of the most common mycotoxins in grains, foods and feeds.[1] deoxynivalenol, as an inhibitor of protein synthesis and as an immunomodulator, can reduce feed intake and mass gain in pigs.[3]
In vitro experiment it shown that treatment with 5 µM deoxynivalenol for 8 h in Pig jejunal cells has the time and dose dependent toxicity responses. In the meanwhile, pig jejunal cells also indicated reduced total cell counts and increased lactate dehydrogenase release after 48 h DON exposure with 0–10 µM. In IPEC-J2 cells, treatment with 20 µM deoxynivalenol at 4, 8, 12 and 24 h also significantly decreased the TEER (trans-epithelial electrical resistance) value.[2] In vitro efficacy study showed that neither 16 μM DOM-1 nor DON concentrations up to 0.4 μM affected the viability of the cells cultivated in medium or ConA. treatment with 0.8 μM deoxynivalenol for cells cultivated in medium significantly increased the count of dead cells, which was even higher at 1.6 μM.[4]
In vivo test demonstrated that mice orally 0.5 to12.5 mg/kg body weight deoxynivalenol decreased liver IGFALS mRNA levels, while 0.1 mg/kg body weight deoxynivalenol is without effect.[3]
References:
[1].Wu S, et al. Detoxification of DON by photocatalytic degradation and quality evaluation of wheat. RSC Adv. 2019 Oct 25;9(59):34351-34358.
[2].Thapa A, et al. Deoxynivalenol and Zearalenone-Synergistic or Antagonistic Agri-Food Chain Co-Contaminants? Toxins (Basel). 2021 Aug 11;13(8):561.
[3].Pestka JJ. Deoxynivalenol-induced proinflammatory gene expression: mechanisms and pathological sequelae. Toxins (Basel). 2010 Jun;2(6):1300-17.
[4].Vatzia E, et al. Deoxynivalenol Affects Proliferation and Expression of Activation-Related Molecules in Major Porcine T-Cell Subsets. Toxins (Basel). 2019 Nov 5;11(11):644.
Average Rating: 5 (Based on Reviews and 37 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















