Dynasore |
| Catalog No.GC10395 |
Dynasore, as a GTPase inhibitor, can rapidly and reversibly inhibit dynamin activity, which prevents endocytosis.
Products are for research use only. Not for human use. We do not sell to patients.
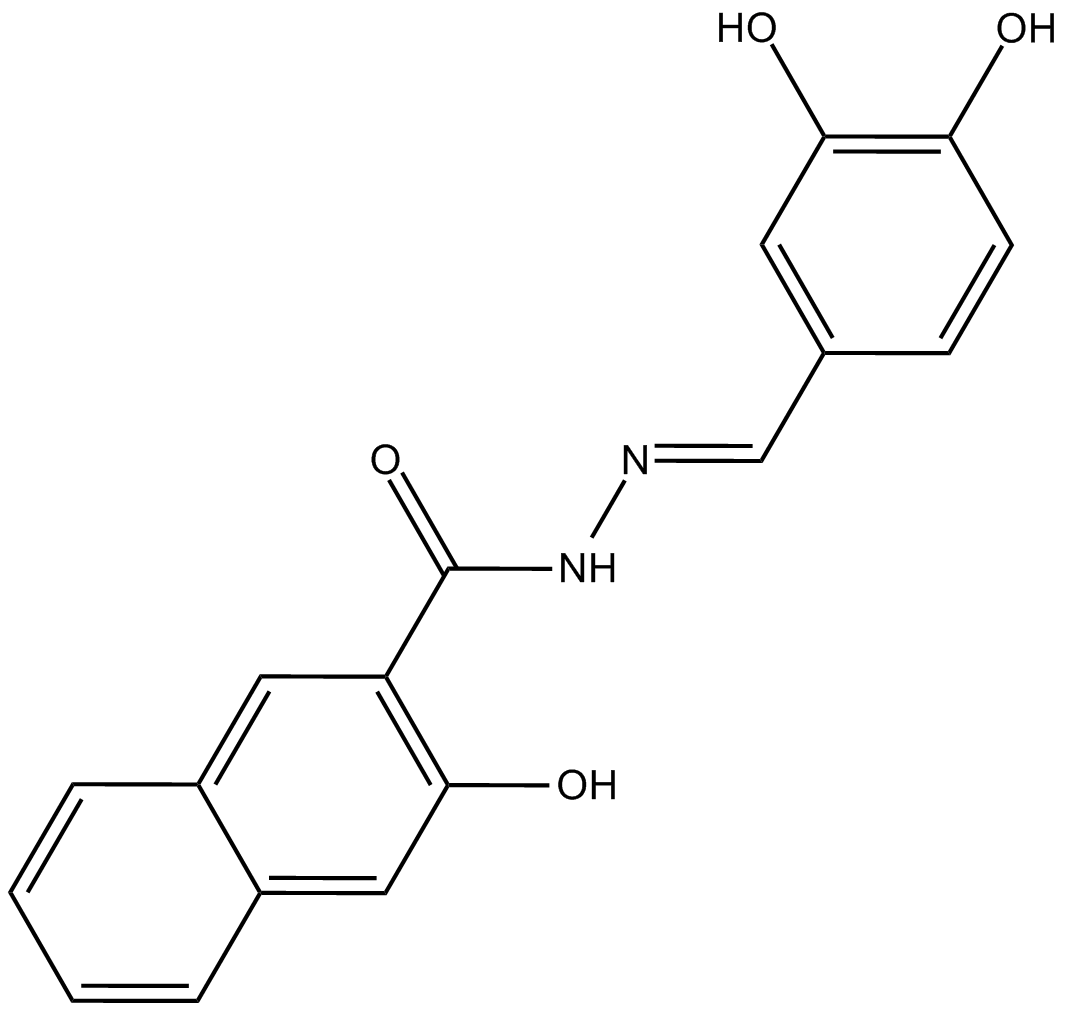
Cas No.: 304448-55-3
Sample solution is provided at 25 µL, 10mM.
Dynasore, as a GTPase inhibitor, can rapidly and reversibly inhibit dynamin activity, which prevents endocytosis[1].
Dynasore inhibits dynamin GTPase activity and transferrin uptake with IC50 of approximately 15 µM[2]. In vitro, treatment with 80 µM dynasore commonly block dynamin 1 and 2, dynasore also has a potent inhibition on ferroptosis at a range of lower concentrations. Dynasore also potently blocked H2O2-induced cell death at 100 nM[3]. In vitro experiment it demonstrated that treatment with 100 µM dynasore impairs VEGF-induced calcium release[4]. At the ocular surface of ex vivo mouse eyes, 40 uM dynasore blocked stress-stimulated dye uptake. Dynasore is obviously protective of cells and their surface glycocalyx, preventing damage due to oxidative stress, and thus precluding dye entry[5]. In vitro test it exhibited that treatment with 100 µM dynasore rapidly increased the spontaneous EPSC (sEPSC) frequency which was followed by inhibition of both solitary tract-evoked EPSCs (ST-EPSC) as well as asynchronous EPSCs[6]. In peritoneal macrophages and LLC-MK2 cells, treatment with 100 µM dynasore drastically diminished the parasite internalization[7].
In vivo efficacy test it shown that dynasore (10 mg/kg, intraperitoneally) inhibits OS tumorigenesis without inducing nephrotoxicity and hepatotoxicity[8]. In vivo, the ocular mouse Sereny model was administrated 30 mg/kg intraperitoneally did not reduce ocular inflammation, it did provide significant protection against weight loss[9].
References:
[1] Preta G, et al. Dynasore - not just a dynamin inhibitor. Cell Commun Signal. 2015 Apr 10;13:24.
[2] Lee S, et al. Synthesis of potent chemical inhibitors of dynamin GTPase. Bioorg Med Chem Lett. 2010 Aug 15;20(16):4858-64.
[3] Clemente LP, et al. Dynasore Blocks Ferroptosis through Combined Modulation of Iron Uptake and Inhibition of Mitochondrial Respiration. Cells. 2020 Oct 9;9(10):2259.
[4] Webster A, et al. Dynasore protects the ocular surface against damaging oxidative stress. PLoS One. 2018 Oct 10;13(10):e0204288.
[5] Hofmann ME, et al. Dynasore blocks evoked release while augmenting spontaneous synaptic transmission from primary visceral afferents. PLoS One. 2017 Mar 30;12(3):e0174915.
[6] Lum M, et al. Impact of dynasore an inhibitor of dynamin II on Shigella flexneri infection. PLoS One. 2013 Dec 19;8(12):e84975.
[7] Zhong B, et al. Dynasore suppresses cell proliferation, migration, and invasion and enhances the antitumor capacity of cisplatin via STAT3 pathway in osteosarcoma. Cell Death Dis. 2019 Sep 18;10(10):687.
[8] Basagiannis D, et al. Dynasore impairs VEGFR2 signalling in an endocytosis-independent manner. Sci Rep. 2017 Mar 22;7:45035.
[9] Barrias ES, et al. Dynasore, a dynamin inhibitor, inhibits Trypanosoma cruzi entry into peritoneal macrophages. PLoS One. 2010 Jan 20;5(1):e7764.
Average Rating: 5 (Based on Reviews and 37 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




