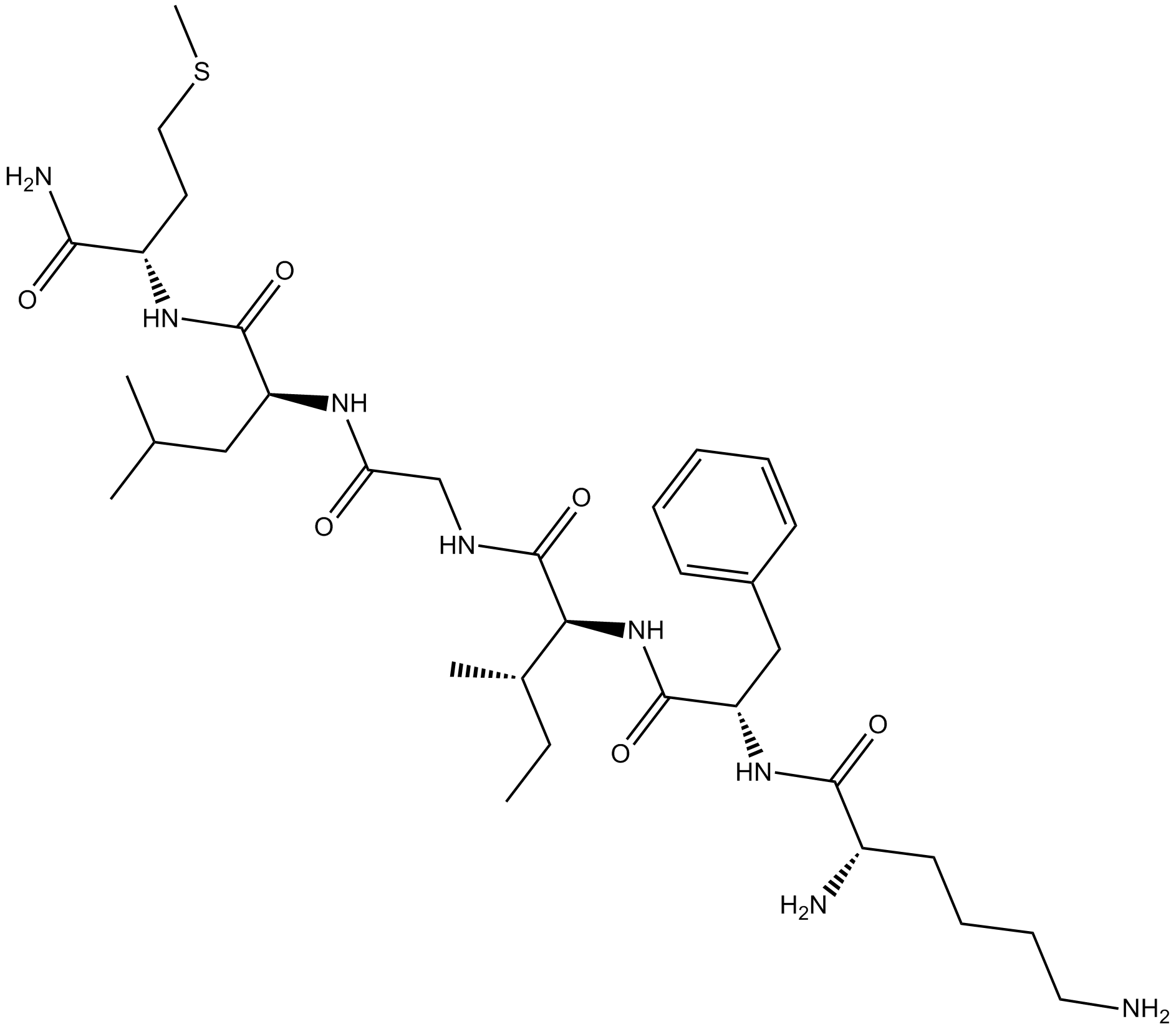
Eledoisin-Related Peptide (Synonyms: Eledoisin Hexapeptide, Eledoisin RP, KFIGLM-NH2, Lys-Phe-Ile-Gly-Leu-Met-NH2) |
| Catalog No.GC15230 |
Tachykinin receptor ligand
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 2990-43-4
Sample solution is provided at 25 µL, 10mM.
Eledoisin Related Peptide is a Substance P analog that excites neurons and triggers behavioral responses. Eledoisin Related Peptide is also a tachykinin receptor ligand.
Eledoisin Related Peptide shares with Substance P (SP) a common N-terminal amino acid sequence and has been shown by to have SP-like activity in the periphery (gut and salivary glands) and the CNS. Eledoisin-related peptide seems to be roughly equipotent with Substance P at identical ejection currents on the single-cell activity of neurons in this nucleus[2]. Both glutamate and substance P (and its analogue, eledoisin-related peptide) have excitatory effects on the activity of respiratory neurons and reflex interneurons[3].
References:
[1]. Severini C, et al. The tachykinin peptide family. Pharmacol Rev. 2002 Jun;54(2):285-322.
[2]. Guyenet PG, et al. Excitation of neurons in the nucleus locus coeruleus by substance P and related peptides. Brain Res. 1977 Nov 4;136(1):178-84.
[3]. Henry JL, et al. Effects of glutamate, substance P and eledoisin-related peptide on solitary tract neurones involved in respiration and respiratory reflexes. Neuroscience. 1985 Mar;14(3):863-73.
Average Rating: 5 (Based on Reviews and 38 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















