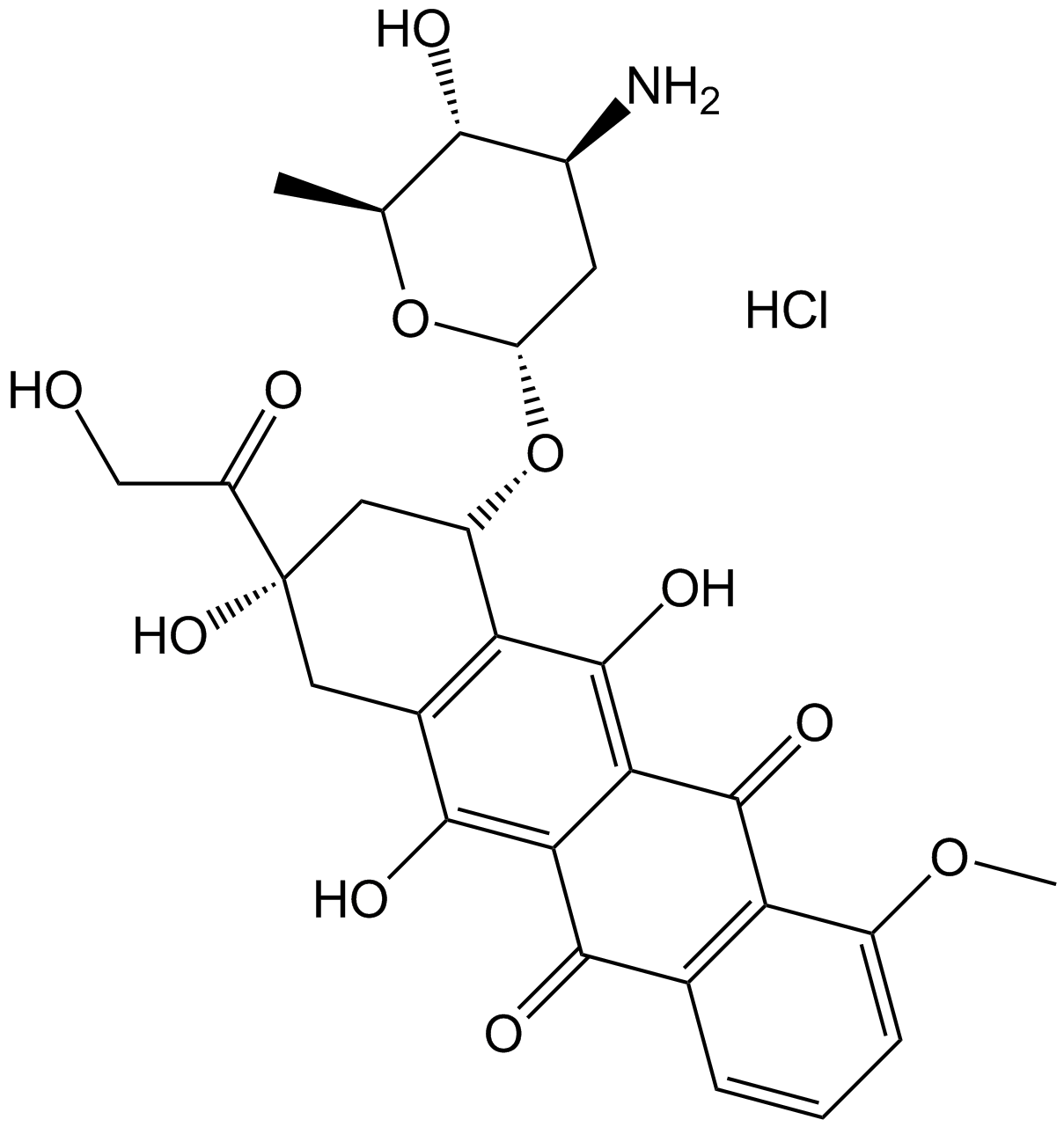
Epirubicin HCl (Synonyms: 4’Epidoxorubicin) |
| Catalog No.GC10686 |
Epirubicin HCl (4'-Epidoxorubicin hydrochloride), a semisynthetic L-arabino derivative of doxorubicin, has an antineoplastic agent by inhibiting Topoisomerase. Epirubicin HCl inhibits DNA and RNA synthesis. Epirubicin HCl is a Forkhead box protein p3 (Foxp3) inhibitor and inhibits regulatory T cell activity.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 56390-09-1
Sample solution is provided at 25 µL, 10mM.
Epirubicin is an inhibitor of DNA topoisomerase (TOPII) [1].
Epirubicin belongs to the Anthracylines chemical class, it is a sort of DNA topoisomerase poison. It can inhibit the religation step of DNA topology, resulting in stabilization of the 5’ phosphotyrosyl-DNA complex (cleavage complex). These lesions are cytotoxic and lead to activation of the DNA damage response and potentially apoptosis. Because of this, it is usually used in cancer therapy. Unfortunately, it also has genotoxic side effects, including the formation of leukemogenic chromosome translocations [1].
Epirubicin is one of the chemotherapeutic agents used for the treatment of Osteosarcoma. It exhibits growth inhibition of tumors by inducing apoptosis. Conversely, it reduces apoptosis in OS cells by activating NF-κB. It is reported that epirubicin combined with cerulenin can enhance the anti-tumor activity in vitro and in vivo [2].
References:
[1] Ian G. Cowell, Caroline A. Austin. Mechanism of Generation of Therapy Related Leukemia in Response to Anti-Topoisomerase II Agents. International Journal of Environmental Research and Public Health. 2012 (9): 2075-2091.
[2] Z.L. LIU, G. WANG, Y. SHU, P.A. ZOU, Y. ZHOU and Q.S. YIN. Enhanced antitumor activity of epirubicin combined with cerulenin in osteosarcoma. Molecular Medicine Reports. 2012 (5): 326-330.
Average Rating: 5 (Based on Reviews and 32 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















