Etamicastat (BIA 5-453) |
| Catalog No.GC32575 |
Etamicastat (BIA 5-453) (BIA 5-453) is a potent and reversible dopamine-β-hydroxylase (DBH) inhibitor with an IC50 value of 107 nM.
Products are for research use only. Not for human use. We do not sell to patients.
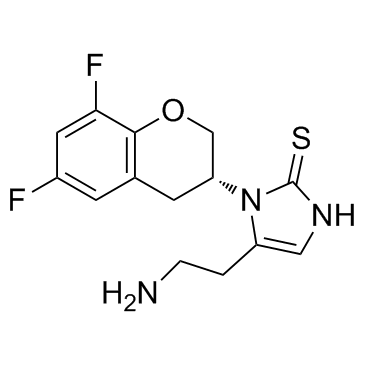
Cas No.: 760173-05-5
Sample solution is provided at 25 µL, 10mM.
Etamicastat (BIA 5-453) is a potent and reversible peripheral dopamine-β-hydroxylase (DβH) inhibitor[1].
Upon intraperitoneal administration to NMRi mice, Etamicastat (100 mg/ kg) leads to a significant reduction of noradrenaline levels (36% control) in heart with concomitant increasing in dopamine levels (850% of control)[2].Etamicastat (50 mg/kg) is rapidly absorbed into the systemic circulation reaching a maximum concentration at 4 h post-administration, representing 29% of total Etamicastat and quantified metabolites, using AUC0-t as a measure of systemic exposure[2].
[1]. Vaz-da-Silva M, et al. Effect of food on the pharmacokinetic profile of Etamicastat (BIA 5-453). Drugs R D. 2011;11(2):127-36. [2]. Loureiro AI, et al. Etamicastat, a new dopamine-?-hydroxylase inhibitor, pharmacodynamics and metabolism in rat. Eur J Pharmacol. 2014 Oct 5;740:285-94.
Average Rating: 5 (Based on Reviews and 22 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















