Ethyl 3-Aminobenzoate (methanesulfonate) (Synonyms: Ethyl m-Aminobenzoate, NSC 93790, Tricaine methanesulfonate, MS-222) |
| Catalog No.GC15824 |
Ethyl 3-Aminobenzoate (methanesulfonate) (MS-222) is common used to immobilize fish for marking or transport and to suppress sensory systems during invasive procedures.
Products are for research use only. Not for human use. We do not sell to patients.
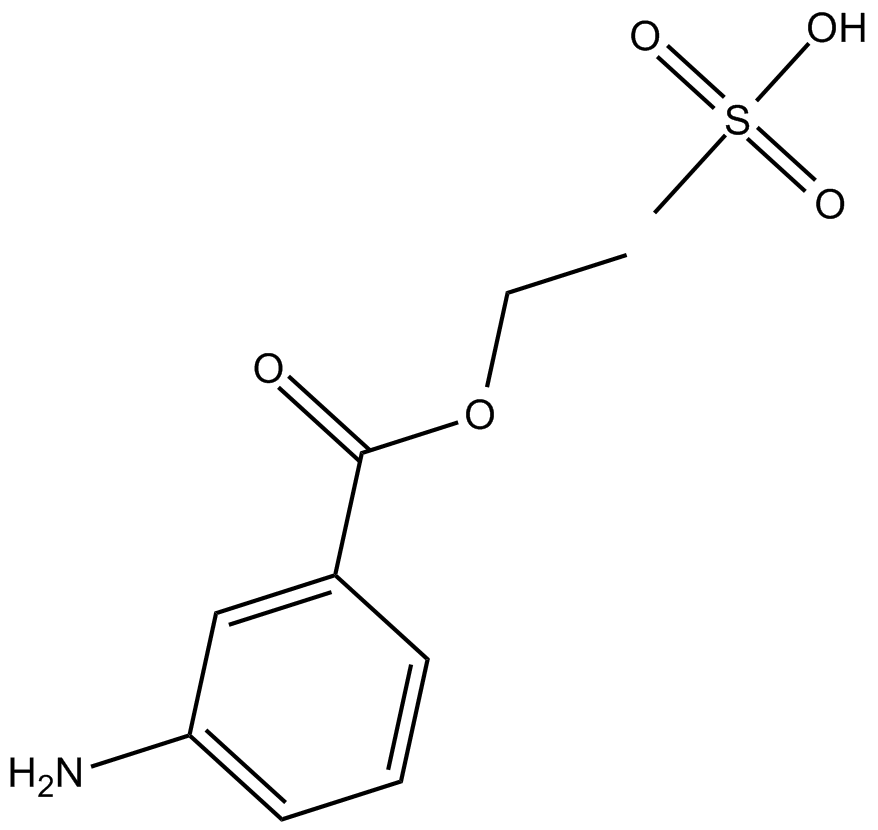
Cas No.: 886-86-2
Sample solution is provided at 25 µL, 10mM.
Ethyl 3-Aminobenzoate can block the generation of action potentials via voltage-dependent Na+-channels.
Sodium channels play a critical role in physiology: they can rapidly transmit depolarizing impulses throughout cells and cell networks, therefore enabling co-ordination of higher processes. Sodium channels are also of special importance for the history of physiology.
In vitro: The major mode of action for ethyl 3-aminobenzoate was found to be nervous system suppression whereby the entrance of sodium into the nerve was inhibited, therefore limiting nerve membrane excitability. In addition, the nerve inhibition was facilitated by the lipid solubility of ethyl 3-aminobenzoate, which allowed it to move easily into the cell membrane to bind with sodium channels [1].
In vivo: Previous study evaluated the potency and dynamics of ethyl 3-aminobenzoate-induced effects on neuronal firing of sensory and motor nerves alongside a defined motor behavior in Xenopus laevis tadpoles. Electrophysiological recordings of extraocular motor discharge and nerve activity were measured before, during and after administration of drugs. Results showed that ethyl 3-aminobenzoate and benzocaine, but not pancuronium could cause a dose-dependent, reversible blockade of extraocular motor and sensory nerve activity. Such results indicated that ethyl 3-aminobenzoate was able to block the activity of both sensory and motor nerves compatible with the mechanistic action of effective anesthetics, suggesting that ethyl 3-aminobenzoate was effective as single-drug anesthetic for surgical interventions in anamniotes [2].
Clinical trial: So far, no clinical study has been conducted.
References:
[1] K. M. Carter, C. M. Woodley and R. S. Brown. A review of tricaine methanesulfonate for anesthesia of fish. Rev. Fish Biol. Fisheries 21, 51-59 (2011).
[2] C. Ramlochansingh, F. Branoner, B. P. Chagnaud, et al. Efficacy of tricaine methanesulfonate (MS-222) as an anesthetic agent for blocking sensory-motor responses in Xenopus laevis tadpoles. PLoS One 9(7), e101606 (2014).
Average Rating: 5 (Based on Reviews and 22 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















