FLAG tag Peptide (Synonyms: H-Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys-OH) |
| Catalog No.GP10150 |
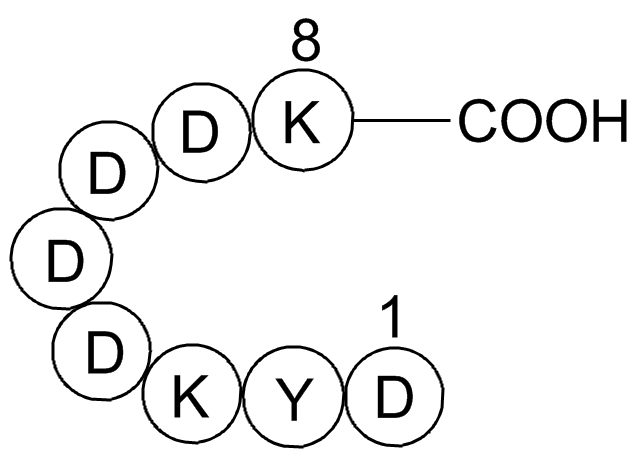
FLAG tag Peptide is an 8-peptide (Asp-Tyr-lys-Asp-Asp-Asp-ASP-ASP-Lys) containing an intestinal kinase restriction site.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 98849-88-8
Sample solution is provided at 25 µL, 10mM.
FLAG tag Peptide is an 8-peptide (Asp-Tyr-lys-Asp-Asp-Asp-ASP-ASP-Lys) containing an intestinal kinase restriction site. FLAG Tag peptide fusion system is used in immunoaffinity chromatography for protein identification and purification. FLAG tag Peptide label peptides allow elution under non-denatured conditions[1,6]. The FLAG tag Peptide can be fused to either the N- or C-terminus of a given fusion protein.
The FLAG tag Peptide marker peptide fusion system comprises a unique and widely useful technique for protein identification and purification. When covalently attached to a solid support, the anti-Flag M1 antibody can be used for the rapid purification of FLAG tag Peptide fusion proteins in a mild, calcium-dependent affinity chromatography procedure. FLAG tag Peptide fusion proteins are typically purified to homogeneity in a single step, starting from a crude cell homogenate or supernatant, without ever exposing the protein to conditions other than physiological saline at pH 7.2 (with calcium or EDTA). The entire purification process can be carried out within several hours[2-3].
The N-terminal FLAG tag Peptide fusion protein can be purified by agarose affinity gel[5]. A short-cut protein purification procedure, where mechanical cell disintegration and affinity purification by immobilization of the anti-Flag M1 antibody on magnetic glass beads are combined. The modified glass beads were added to yeast cells and mixed on a laboratory vortex mixer. The magnetic glass beads capture the target protein, while the yeast cells are disrupted. By making contact with a stationary magnet, the magnetic glass beads can very efficiently be separated from yeast cell debris. Chelating agents, such as EDTA, were capable of eluting the FLAG tag Peptide fusion proteins from the magnetic glass beads[4].
References:
[1]. Einhauer A, Jungbauer A. The FLAG peptide, a versatile fusion tag for the purification of recombinant proteins. J Biochem Biophys Methods. 2001 Oct 30;49(1-3):455-65. doi: 10.1016/s0165-022x(01)00213-5. PMID: 11694294.
[2]. Chiang CM, Roeder RG. Expression and purification of general transcription factors by FLAG epitope-tagging and peptide elution. Pept Res. 1993 Mar-Apr;6(2):62-4. PMID: 7683509.
[3]. Prickett KS, Amberg DC, Hopp TP. A calcium-dependent antibody for identification and purification of recombinant proteins. Biotechniques. 1989 Jun;7(6):580-9. PMID: 2698650.
[4]. Schuster M, Wasserbauer E, Ortner C, Graumann K, Jungbauer A, Hammerschmid F, Werner G. Short cut of protein purification by integration of cell-disrupture and affinity extraction. Bioseparation. 2000;9(2):59-67. doi: 10.1023/a:1008135913202. PMID: 10892539.
[5]. Hopp TP, Prickett KS, Price VL, et al. A Short Polypeptide Marker Sequence Useful for Recombinant Protein Identification and Purification. Bio/technology (Nature Publishing Company). 1988;6:1204-1210. DOI: 10.1038/nbt1088-1204.
[6].Li Y. Commonly used tag combinations for tandem affinity purification. Biotechnol Appl Biochem. 2010 Feb 15;55(2):73-83. doi: 10.1042/BA20090273. PMID: 20156193.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




