Fluorouracil (Adrucil) (Synonyms: 5-FU, NSC 19893, Ro 29757, U8953) |
| Catalog No.GC14466 |
A prodrug form of FdUMP
Products are for research use only. Not for human use. We do not sell to patients.
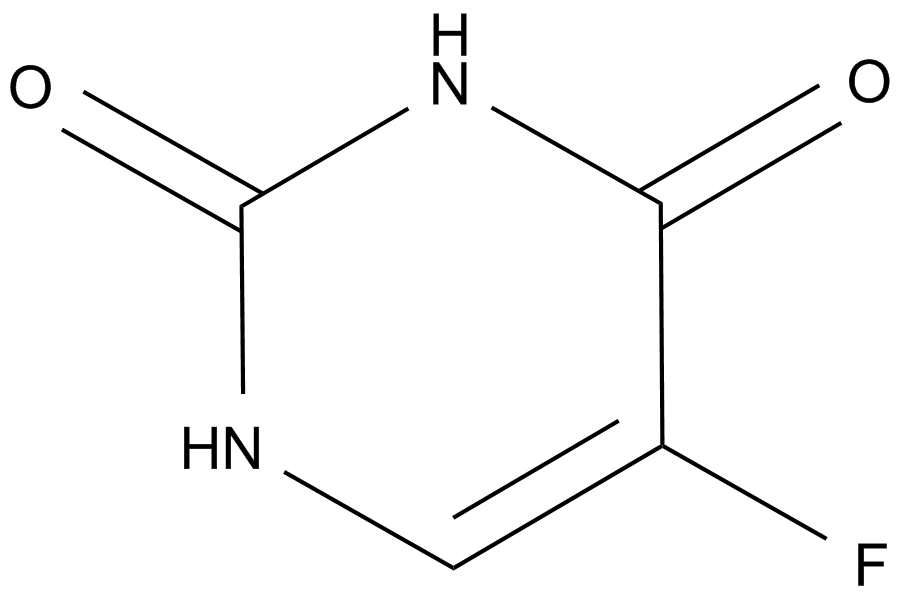
Cas No.: 51-21-8
Sample solution is provided at 25 µL, 10mM.
Fluorouracil (Adrucil) (5-FU) is an analogue of uracil with a fluorine atom at the C-5 position in place of hydrogen [1]. Fluorouracil is widely used in the treatment of a range of cancers, including colorectal and breast cancers, and cancers of the aerodigestive tract [1].
The mechanism of cytotoxicity of Fluorouracil (Adrucil) has been ascribed to the misincorporation of fluoronucleotides into RNA and DNA and to the inhibition of the nucleotide synthetic enzyme thymidylate synthase (TS) [1]. Fluorouracil (Adrucil) at the concentration of 770µM treated HCT116 parental cell line and its 5-FU-resistant derivatives, Fluorouracil (Adrucil) led to a G1(/S) arrest at 8 and 24 hours [2]. The G1 arrest was most pronounced in ContinD cells at 24 hours, whereas the S phase arrest was most pronounced in parental HCT116 cells at 24 hours [3].
Fluorouracil (Adrucil) beneficial effected in intestinal tumorigenesis in the Apcmin/+ mice model where a 60-80% reduction in polyps [4]. Fluorouracil (Adrucil) reduces body weight, exacerbates symptom severity score, increases liver weight, and decreases epididymal fat mass, and decrease in survival [5].
References:
[1]. Longley D B, Harkin D P, Johnston P G. 5-fluorouracil: mechanisms of action and clinical strategies[J]. Nature reviews cancer, 2003, 3(5): 330-338.
[2]. Takeda H, Haisa M, Naomoto Y, Kawashima R, Satomoto K, Yamatuji T, Tanaka N: Effect of 5-fluorouracil on cell cycle regulatory proteins in human colon cancer cell line. Jpn J Cancer Res. 1999, 90: 677-684.
[3]. De Angelis P M, Svendsrud D H, Kravik K L, et al. Cellular response to 5-fluorouracil (5-FU) in 5-FU-resistant colon cancer cell lines during treatment and recovery[J]. Molecular cancer, 2006, 5(1): 1-25.
[4]. J.M. Tucker, C. Davis, M.E. Kitchens, et al. Response to 5-fluorouracil chemotherapy is modified by dietary folic acid deficiency in Apc(Min/+) mice. Cancer Lett., 187 (2002), pp. 153-162
[5]. Sougiannis A T, VanderVeen B N, Enos R T, et al. Impact of 5 fluorouracil chemotherapy on gut inflammation, functional parameters, and gut microbiota[J]. Brain, behavior, and immunity, 2019, 80: 44-55.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




