5-fluoro 203 (Synonyms: 5F-203,NSC 703786) |
| Catalog No.GC12209 |
Le 5-fluoro 203 (NSC-703786) est une molécule cytotoxique qui forme des adduits À l'ADN et arrête le cycle cellulaire.
Products are for research use only. Not for human use. We do not sell to patients.
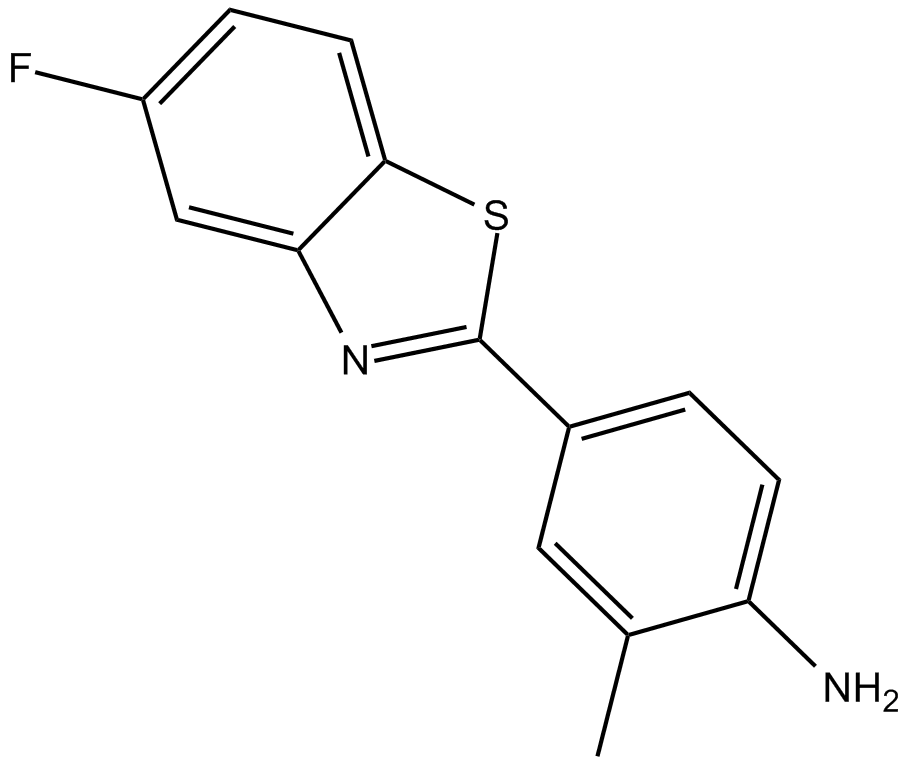
Cas No.: 260443-89-8
Sample solution is provided at 25 µL, 10mM.
5-fluoro 203 (5F-203) is an antitumor agent and cytotoxic compound that acts as a potent AhR agonist [1][2][3].
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor involved in regulating xenobiotic-metabolizing enzymes such as cytochrome P450.
5-fluoro 203 (5F-203) is an antitumor agent that acts as a potent AhR agonist. In MCF-7 breast cancer cell, 5-fluoro 203 inhibited cell growth in part by activating the aryl hydrocarbon receptor (AhR) signaling pathway. 5-fluoro 203 increased protein-DNA complex formation on the NF-kB-responsive element and also increased NF-kB-dependent transcriptional activity [1]. In sensitive MCF-7 cells, 1.0 μM 5F-203 induced CYP1A1 gene expression [2]. In IGROV-1 cells, 5F203 induced enhanced CYP1A1 expression, AhR translocation and ROS formation, accompanied by JNK, ERK and P38 MAPK phosphorylation, DNA damage and cell cycle arres. In cells isolated from ovarian cancer ascites, 5F203 also induced CYP1A1 expression, AhR translocation and ROS formation [3].
In nude mice bearing human tumor xenografts, 5F-203 induced CYP1A1 gene expression, which was correlated with in vivo sensitivity. Therefore, induction of CYP1A1 mRNA in response to 5F-203 treatments may provide a possible surrogate marker for determination of drug-sensitive tumors in patients [2].
References:
[1]. Brantley E, Patel V, Stinson SF, et al. The antitumor drug candidate 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole induces NF-kappaB activity in drug-sensitive MCF-7 cells. Anticancer Drugs. 2005 Feb;16(2):137-43.
[2]. Hose CD, Hollingshead M, Sausville EA, et al. Induction of CYP1A1 in tumor cells by the antitumor agent 2-[4-amino-3-methylphenyl]-5-fluoro-benzothiazole: a potential surrogate marker for patient sensitivity. Mol Cancer Ther. 2003 Dec;2(12):1265-72.
[3]. Callero MA, Luzzani GA, De Dios DO, et al. Biomarkers of sensitivity to potent and selective antitumor 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole (5F203) in ovarian cancer. J Cell Biochem. 2013 Oct;114(10):2392-404.
Average Rating: 5 (Based on Reviews and 29 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















