How to Choose the Correct Dosing Method for Mice? Gavage VS Intraperitoneal VS Intravenous?
There are various modes of administration in animal experimental studies, such as oral, intraperitoneal, intravenous, intracerebral injection, etc. However, we often choose a certain drug delivery method commonly used in the laboratory without knowing the reason. For example, our laboratory often chooses intraperitoneal injection, but do not know why. In fact, there is a lot of knowledge included! In this case, from the perspective of pharmacology and pharmacokinetics, we will popularize the principles of choosing the mode of administration.
1.Commonly used drug delivery methods for laboratory animals
Common administration methods for experimental animals are: oral, intravenous, intraperitoneal, subcutaneous, intramuscular, intraspinal, intraventricular, intradermal, lymphatic, nasal and sublingual..
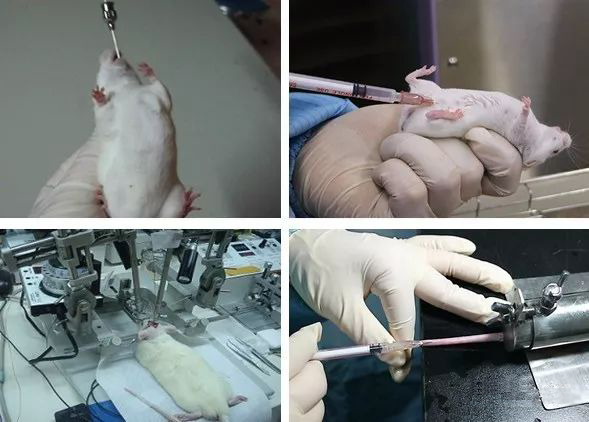
Operation diagram of common various drug delivery methods (respectively: gavage, intraperitoneal, intracerebral and tail vein injection)
1.1. Oral administration (po)
• Drug circulation route: esophagus → stomach → small intestine → small intestinal capillaries (absorption into blood) → jejunal vein → superior mesenteric vein → hepatic portal vein → liver → hepatic vein → inferior vena cava → right atrium → right ventricle absorption into the blood circulation;
• Features: safe and convenient; absorbed through the gastrointestinal tract, with first-pass metabolism in the gastrointestinal tract and first-pass metabolism in the liver, and the bioavailability of some compounds will be very low;
• Difficulty of administration: mainly refers to gavage, the operation is relatively simple;
• Dosing volume: mouse: 0.1-0.8ml; rat: 1-3ml.
1.2. Intraperitoneal administration (ip)
• Drug circulation route: superior mesenteric vein → hepatic portal vein → liver → hepatic vein → inferior vena cava → right atrium → right ventricle absorption into blood circulation;
• Features: There is no first-pass metabolism in the gastrointestinal tract, but there is still first-pass metabolism in the liver, and the bioavailability is high;
• Difficulty of administration: relatively simple;
• Dosing volume: mice: 0.2-0.8ml; rats: 1-2ml.
1.3. Intravenous (iv)
• Drug circulation route: the drug directly enters the blood;
• Features: There is no absorption barrier and first-pass metabolic barrier, and the bioavailability is the highest;
• Difficulty of administration: difficult;
• Dosing volume: Mice: 0.05-0.2ml.
1.4. Subcutaneous injection (sc) and intramuscular injection (im)
The drug is mainly absorbed through subcutaneous and intramuscular capillaries. The absorption is not as complete as intravenous injection, but subcutaneous injection and intramuscular injection also successfully avoid the gastrointestinal tract and liver. first-pass metabolism;
• Dosing volume: mouse: 0.05-0.1ml; rat: 0.1-0.2ml.
1.5. Intradermal injection (id)
Intradermal injection is to inject the liquid medicine between the epidermis and dermis. Like some antibiotics for allergic skin tests, it is intradermal injection, and the absorption of intradermal injection is slow;
• Dosing volume: mouse: <0.05ml; rat: <0.1ml.
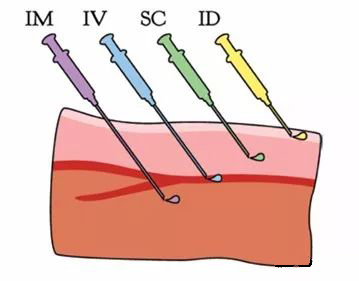
Administration site for four commonly used injection methods[1]
1.6. Intracerebral Injection
As shown in the figure above, the coordinates of the locator are adjusted according to the stereoscopic brain map, and the drug is precisely injected into the cerebral ventricle or specific tissues of the brain, such as the hippocampus, substantia nigra, etc.
• Features: Directly inject the drug into the brain, skip the drug absorption and the blood-brain barrier, and ensure the amount of the drug entering the brain [2]; the volume of intracerebral injection is very small, <0.03ml.
After understanding the characteristics of the above-mentioned various modes of administration, how should we choose in experimental animal research?
2. Choose according to the purpose of the experiment
Animal experiments can be roughly divided into efficacy experiments, pharmacokinetics experiments, toxicity experiments and toxicity experiments.
2.1. Pharmacodynamic experiment: The drug should be selected with reference to the proposed clinical administration method of the developed drug and the indications; for example, intravenous and intraperitoneal injections have fast absorption rates and are suitable for emergency treatment.
2.2. Pharmacokinetic experiments: From the perspective of bioavailability, if you want to study oral bioavailability, use animal gavage, and if you want to study intraperitoneal bioavailability, use intraperitoneal administration;
2.3. Toxicity experiments: Especially acute toxicity experiments, because different routes of administration have different drug exposures and absorption rates, the toxicity of compounds varies greatly.
The following table shows the bioavailability of various modes of administration. Intravenous administration is the highest, followed by: intraperitoneal > intramuscular > subcutaneous > oral; to sum up, we can choose a reasonable administration method based on the difference in bioavailability [3].

3.Further combination of drug properties
Mainly consider the solubility, stability and toxicity of the drug; the mode of administration selected according to the purpose of the experiment may not support your mode of administration due to the nature of the drug. For example, when choosing intravenous injection, it is found that the drug is difficult to dissolve or oral administration has strong gastrointestinal damage.
3.1. Solubility: Drugs often mainly include small molecular compounds, natural extracts and Chinese herbal medicines, etc. The differences in the physicochemical properties of drugs often lead to different solubility in media. Water is the best reagent, but many synthetic small molecule compounds are insoluble in water, we need to find a suitable medium, and finally refer to the dosage forms available for various administration methods.
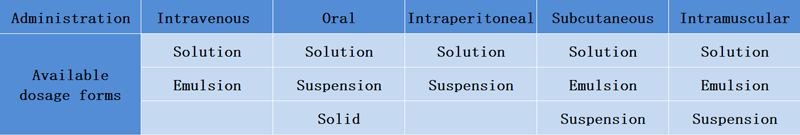
The following table shows the acceptable pharmaceutical dosage forms for commonly used administration methods:
Remark:
• Oral administration: There are many optional dosage forms, as long as the drug is uniform and stable.
• Intravenous administration: There are relatively high requirements on the dosage form, and the solution that needs to be completely dissolved or the system in which the drug particles are much smaller than red blood cells, such as oil-in-water emulsion, etc. [4], therefore, the insoluble dosage form cannot be considered for intravenous injection;
• Intracerebral and subcutaneous injection: Intracerebral and subcutaneous doses are small and need to be combined. For example, under the same dosage, the drug concentration prepared by intracerebral injection and subcutaneous injection will be higher. At this time, it is necessary to consider whether the solubility of the drug meets the requirements;
3.2. Stability: For example, the gastrointestinal tract in animals has a certain pH value, and some sections are very acidic. If the compound has pH instability in vivo, it should be administered in a way other than oral administration;
3.3. Toxicity: If a drug is found to have enterotoxicity when taken orally, other methods of administration such as intravenous can be considered to avoid the drug's absorption through the intestines; the possibility of enterotoxicity of traditional Chinese medicine ingredients is relatively high, so when developing active compounds of traditional Chinese medicines Vigilance is required.
4. Finally, consider the R&D cost and technical difficulty
For example, in practice, in the early stage of compound screening, it will not invest too much money and time to improve the solubility and other properties of the compound, so try to choose an acceptable dosage form corresponding to the administration method; When long-term intravenous administration is difficult to operate, it may be considered to choose intraperitoneal administration with a slightly worse bioavailability than intravenous administration for experiments.
From the perspective of new drug development, oral administration is the best!
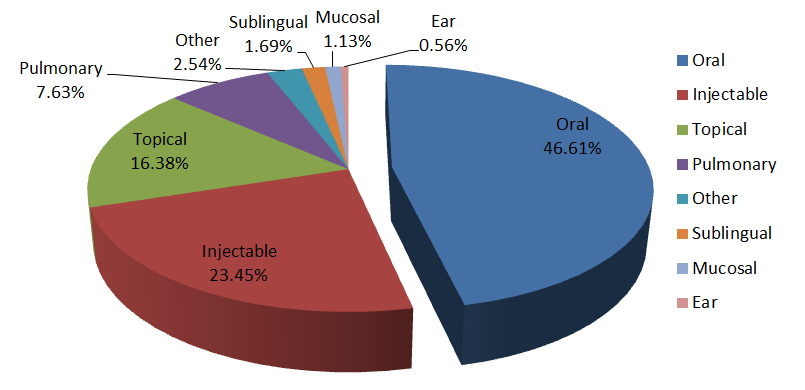
The following figure shows the distribution of new drug preparations approved by the FDA from 2004 to 2013. Among them, oral administration accounted for the largest proportion, accounting for 46.61%, followed by injection administration.
2004-2013 FDA-approved new drug administration routes[5]
Because oral administration is the most convenient, economical and safe way, it accounts for the largest proportion. From this, there is a saying that the drugs that can be taken orally are never intravenous, because intravenous drugs not only have high research and development costs, but also have high risks. The same strategy is also applicable to experimental animals. However, not all drugs or conditions are suitable for oral administration. In specific experimental studies, we should choose the most appropriate way of administration.
In summary, we briefly introduce some strategies or principles for the selection of drug delivery methods in experimental animal research. This article is a summary of my usual experience. If there are discrepancies or any questions, you are welcome to raise them and learn from each other.













Commentaires