DOXORUBICIN
Dox or DOX is the short form of Doxorubicin, which is also called as Adriamycin,
The molecular structure of Doxorubicin is diagrammatically depicted in Figure 1. In its molecular structure, there is a tetracyclic quinoid aglycone adriamycinone namely 14-hydroxydaunomycinone that is chemically linked to the amino- sugar namely daunosamine. Chemically, it is a derivative of Anthracycline that has antibiotic as well as anticancer properties. The amino group along with the quinone moiety present in the molecular structure of Doxorubicin are the main source of determining the anti-cancer properties of Doxorubicin.
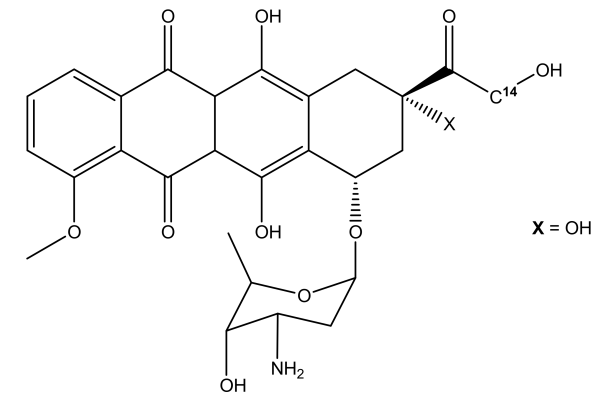
Figure 1 The Molecular Structure of Doxorubicin
Although the mode of action of Doxorubicin in the human body is not fully understood, there are some views are presented that are supported by the scientific literature. Several events are found to be associated with the anticancer/ cytotoxic effects of Doxorubicin. These events include: topoisomerase II gets permanently inhibited via enzyme poisoning, cytochrome C get released from the mitochondria, small Doxorubicin molecules get intercalated in the major and minor grooves of the DNA double helical structure, DNA get uncoiled, alkylation gets induced, free radicals are produced in large amount thus oxidative stress is induced. The core mechanism of action that Doxorubicin demonstrates includes the intercalation of small Doxorubicin molecules into the double helical structure of the DNA molecule. This intercalation happens only because the Doxorubicin molecule have the sugar moieties as well as the cyclohexane ring which is clearly seen from the molecular structure of Doxorubicin given in Figure 1. As a result of this intercalation, a stable DOX-DNA complex is formed which is schematically shown in Figure 2 and thereby the DNA replication as well as DNA transcription is prevented as the activity of the topoisomerase II enzyme is hampered. Moreover, this intercalated DNA molecule is prone to damage and thus leads the cell to the cell death processes.
The structural features of the Doxorubicin molecule, owing to which the DOX-DNA complex gets formed are indicated in Figure 3. While, the core stabilizing interactions for this complex are the Van der Waal forces. As DOX-DNA complex is formed, histones are evicted from their location. When located properly, Histones are involved in assembling, spatial organizing and compacting the chromatin which is essential for the normal nuclear processes, like replication and transcription. When these histones are evicted, new histones with different epigenetic marks takes that place. This leads to a cascade of events including epigenetic and transcriptomic alterations. All of them are collectively called as chromatin damage.
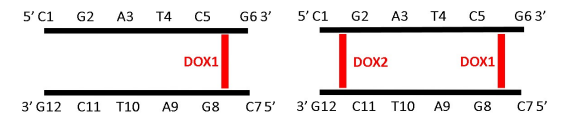
Figure 2 The DOX-DNA Complex. Many DOX molecules can get intercalated between two complementary strands of a DNA molecule. Here in the figure, DOX1 is the first molecule that do intercalation which DOX2 is the second one
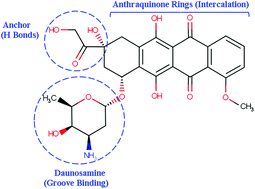
Figure 3 The structural features of the Doxorubicin molecule that takes parts in the intercalation between two complementary DNA strands include the Anthraquinone rings, Daunosamine and the anchor which are indicated by blue. Daunosamine binds to the grooves of the DNA double helix, while the anchor part/ segment gets attracted to the H bonds of the DNA strands
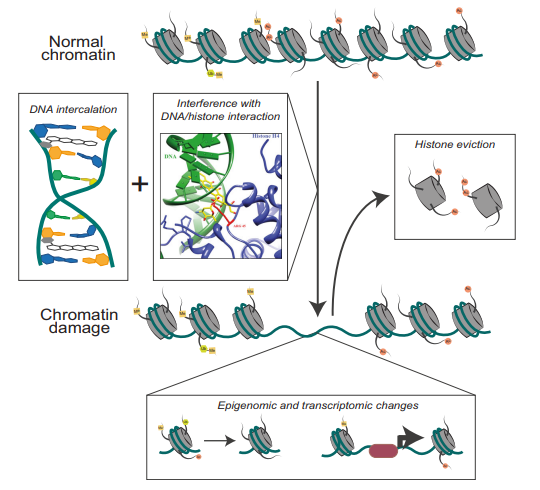
Figure 4 The chromatin damage occurs due to the intercalation of Doxorubicin molecules and thereby eviction of histone proteins
It is found that in some cases, the Doxorubicin molecules damages the mitochondrial DNA as well in addition to the nuclear DNA through the same mode of action as explained previously. There is also a secondary mode of action that is proposed which states that Doxorubicin treatment leads to the excessive production of the Reactive Oxygen Species by the treated cells. In this mechanism, first the Doxorubicin molecules are chemically oxidized to a semiquinone. Semiquinone is inherently an unstable metabolite, which means that it is rapidly metabolized by the body as soon as it is formed inside the body. This semiquinone is converted back into Doxorubicin by the body’s metabolism, and as a by-product Reactive Oxygen Species are formed.The Reactive Oxygen Species includes hydrogen peroxide (H2O2) and superoxide anions (O2−), which are converted into free radicals namely hydroxyl radical OH.. These free radicals damages all the core structural as well as the core functional molecules of the cell through different processes/ mechanisms, i.e., the lipid molecules undergo peroxidation, the protein molecules undergo denaturation, the DNA molecule undergo mutation. In short, the free radicals have all kinds of deleterious effects on the cells and thus triggers the apoptosis that is a type of a programmed cell death. Both of these modes of actions of Doxorubicin are summarized and diagrammatically shown in Figure 4.
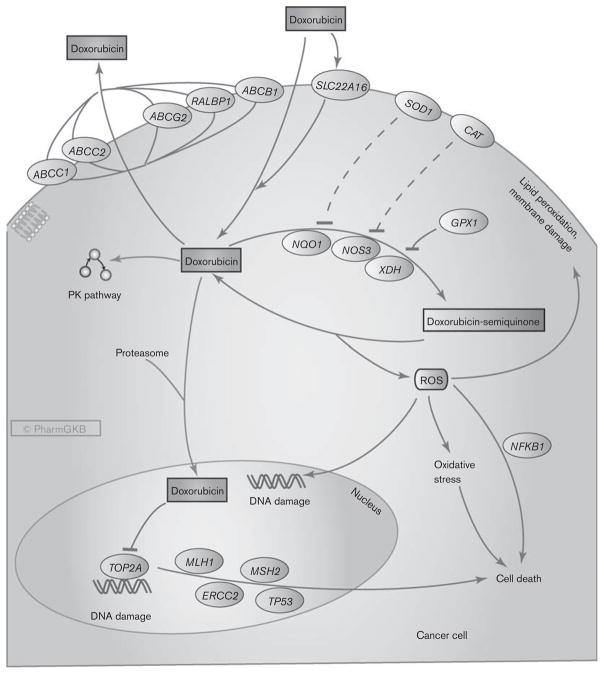
Figure 5 Two Modes of Actions of Doxorubicin: First is the intercalation between the complementary strands of the DNA dimer, while second is the excessive production of the Reactive Oxygen Species ROS.
Another mechanism that is proposed to describe the anti-tumor functionality of Doxorubicin; it states that Doxorubicin causes an increase in the level/ concentration of ceramides inside the cells and thereby a membrane transcription factor is released namely CREB3L1. This transcription factor, CREB3L1, affects expression of multiple genes via disturbing their transcription. One of the affected gene is p21; which is inherently a tumor suppressor gene. It is also proposed that only those tumors show sensitivity to Doxorubicin which are having elevated levels of this transcription factor.
Doxorubicin demonstrates a cell cycle specific activity, that means that it hinders the cell cycle at the specific stages only. Doxorubicin causes G0/S and G2/M arrest in the tumor cells.
Doxorubicin is a pharmaceutical drug that has been commonly used as a chemotherapeutic agent against different types of cancers/ neoplasm in the clinical settings, owing to its anti-cancer properties. These cancers include bone sarcomas, Hodgkin lymphoma, acute lymphoblastic leukaemia, acute myeloblastic leukaemia and the cancers of organs like thyroid, breast, lungs, bladder and ovary. In addition, the liposomal formulation of Doxorubicin is FDA approved for the treatment of the ovarian cancer. Although, it is an effective anti-cancer drug, its high dosage leads to the major side effects namely toxicity, i.e., cardiotoxicity, renal toxicity/ nephrotoxicity, and gonadotoxicity. These are the toxicity to different bodily organs namely heart, kidneys and gonads that is of testicular and ovarian nature in the males and females, respectively. Apart from these toxicities, there are some limitations that are preventing Doxorubicin to be an ideal drug for chemotherapy against cancer. These limitations are low solubility, shorter half-life and non-specificity.
Due to the potential limitations and toxicities, various types of molecules with better drug properties and lower toxicities, are derived from the basic structure of Doxorubicin molecule which is given in Figure 1. These are called as hybrid derivatives of Doxorubicin. Cholesteryl-Doxorubicin Derivatives, Doxorubicin-Fatty Acyl Derivatives, Doxorubicin-Hydrazone Derivatives, Doxorubicin Hybrid Containing Compounds with Antioxidant Activity, Formamidino-Doxorubicin Derivatives, Dexamethasone-Doxorubicin Derivative, Doxorubicin Derivative Containing Arimetamycin Scaffolds, Photoresponsive-Doxorubicin Hybrid, Steroidal Anti-Estrogen−Doxorubicin Bioconjugate are all such types of hybrid Doxorubicin derivatives.













Commentaires