Leu 15 Gastrin 1 Human
There are various names for the [Leu 15] -Gastrin 1 (Human); these names include human heptadecapeptide, [Leucine15]-Little gastrin I, [Leu15]-HG-17 and also LHG-17.
Biochemically, Human Gastrin 17 is a small peptide that is naturally present in the human digestive system, central nervous system and also in the peripheral nervous system, whose length is just 17 amino acids. The sequence of amino acids AA in the Human Gastrin 17 is diagrammatically presented in Figure 3. It primarily acts as a gut hormone that acts as a trophic hormone thus affecting other endocrine glands that maintains the physiologic release of the gastric acid as well as the normal growth of the fundic mucosa. Therefore, in-short the level of the Gastrin 17 has a homeostatic importance in terms of normal digestion. Either low or high levels are abnormal and can even be lethal. Let us see how the levels of the gastrin can affect the normal health. In the first case, when there is little or very small release of the gastrin, there is not gastrin is available that can secrete enough gastric acid that can kill the bacteria that come along with the consumed food. In that case, the bacteria level of the blood would increase dramatically which would ultimately lead to the metaplasia in the intestines and also to developing tumors that eventually leads to the cancer development. In the second case, when there is very much release of the gastrin is also pathological state, e.g., Zollinger-Ellison syndrome. These pathologies are characterized by the ulcers in the duodenal and jejunal regions of the intestines and marked by the excessive level of gastrin in the blood, in the medical terms this is called as hypergastrinemia. It has been scientifically shown in the animal models that hypergastrinemia is a medical condition that imposes a high risk of cancers/ neoplasms as evident by the presence of the precancerous colonic polyps and mutations of the APC gene. Apart from its disturbed level of secretion, various neoplasms/ cancers are also characterized by the expression of the Gastrin peptide, namely gastric cancer, colorectal cancer, brain tumors, lung cancers, pancreatic cancers and ovarian cancers. In these cancers, Gastrin causes the induction of the carcinogenic tumors. Either causing the down regulation of the Gastrin-17 peptide or interrupting the interactivity between the Gastrin-17 peptide and its receptor protein is considered as a promising treatment strategy for these malignancies. The latter could be achieved by using such antibodies or antagonists for the involved receptor that highly specific in their action.
The production/ biosynthesis and release of the Gastrin-17 peptide is carried out by the mucosal G cells in the antral and duodenal mucosa, as a normal response to the ingestion of food. In addition, this produced Gastrin-17 peptide stimulates the enterochromaffin-like (ECL) cells for the production and release of histamine. On top of that, histamine then finally leads to the induction of the gastric acid release from the parietal cells. The final release of the gastric acid is mediated via the activity of the active H2 histamine receptors.
The biosynthesis of Gastrin-17 peptide takes place in the normal endocrine cells as well as in the tumor cells. Gastrin-17 peptide is produced after a chain of post-translational modifications; firstly, it is coded by gene in the form of a precursor having the length of 101 amino acids which is called as preprogastrin. The preprogastrin has one such peptide that gets the preprogastrin translocated in the Endoplasmic reticulum and that leads to the secretory pathway. The signal peptidase is an enzyme that cleaves the preprogastrin and forms progastrin; by this cleavage progastrin can move out of the Endoplasmic reticulum and then, move in the secretory granules of the Golgi Apparatus. Under normal physiological conditions, progastrin can be biochemically converted into glycine-extended gastrin-34 (gly-G34) or glycine-extended gastrin-17 (gly-G17) by the activity of the enzymes in the secretory granules. Both of these, act as substrates for the peptidylglycine α-amidating monooxygenase (PAM) which brought about their amidation and them into amidated gastrin-34 (G34-NH2) and amidated gastrin-17 (G17-NH2), respectively. The more details of the normal biosynthesis of the Gastrin are diagrammatically presented in Figure 1.
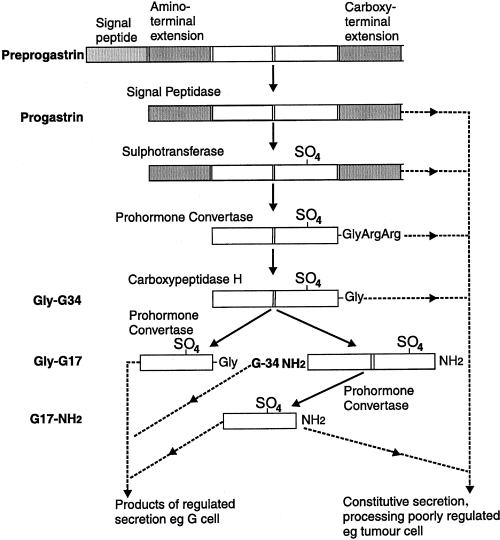
Figure 1 The details of the biosynthesis of Gastrin under the normal physiological conditions: it starts from preprogastrin that gets converted into progastrin. Progastrin may be converted into gly-G34 or gly-G17; this is followed by their final amidation into G34-NH2 and G17-NH2. Am important point to note is that all of these molecules formed are the precursors of the gastrin 17 polypeptide
The gastrin 17 polypeptide affect the tumor growth via endocrine as well as autocrine and paracrine pathways. It acts via the endocrine pathway is scientifically shown; in those experiments when the growth was increased in the in vitro cultures of the tumor cell lines particularly colorectal cancer, when treated with gastrin 17 polypeptide. It acts via the autocrine and the paracrine pathways is also scientifically shown; especially in those experiments where it is clearly observed that the tumor cells themselves express the gastrin 17 polypeptide as well as its respective receptor. This expression of the gastrin 17 polypeptide and its respective receptor is slightly different from the normal physiological biosynthesis of the gastrin 17 polypeptide, which is summarized in Figure 1 . This difference is evident as there is a high concentration of the preprogastrin formed but a low concentration of the mature peptides, however it is also observed in some cell lines that the mature peptides do form in the cancerous cells as well. All the involved pathways are summarized and diagrammatically presented in Figure 2.
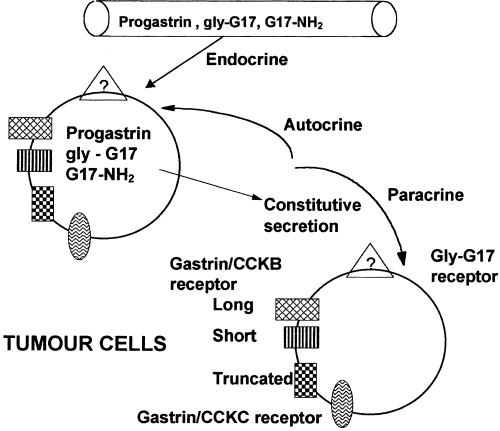
Figure 2 The modes of action of the Gastrin: it happens through endocrine, autocrine and paracrine manner
The receptor protein of the Gastrin-17 peptide is CCK2 receptor (CCK2R), which is traditionally called as CCK-B receptor the short form of cholecystokinin-B receptor.

Figure 3 The Sequence of Amino Acids AA in the Gastrin-17 peptide is given in the terms of three letter code
In the sequence of the amino acids in the Gastrin-17 peptide, which is given in Figure 3. The methionine residual at the position 15 gets oxidized and thereby all of its biological function is lost, which is to stimulate the secretion of the Gastric acid. Therefore, several molecular analogues of the Gastrin-17 peptide are produced in the laboratory. The primary thing in all these molecular analogues is that the methionine residual at the position 15 is substituted with any other amino acid. [Leu 15] -Gastrin 1 is one of such synthetic molecular analogues that was created to retain all the biological functions that Gastrin-17 peptide normally do by protecting against the oxidation.
Leu 15 Gastrin 1 is a synthetic peptide and the laboratory method to produce Leu 15 Gastrin 1 and how to handle it, is fully documented in detail in the published scientific literature.














Commentaires