LOXO-101 (Larotrectinib) (Synonyms: ARRY-470, Larotrectinib) |
| Catalog No.GC36425 |
LOXO-101 (Larotrectinib) (LOXO-101) is an ATP-competitive oral, selective inhibitor of the tropomyosin-related kinase (TRK) family receptors, with low nanomolar 50% inhibitory concentrations against all three isoforms (TRKA, B, and C).
Products are for research use only. Not for human use. We do not sell to patients.
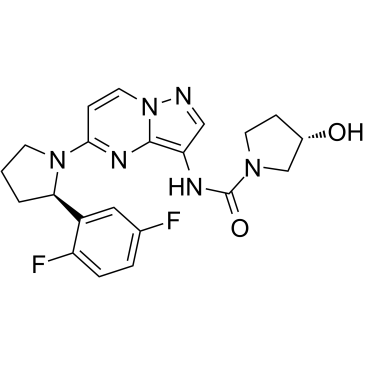
Cas No.: 1223403-58-4
Sample solution is provided at 25 µL, 10mM.
Larotrectinib (LOXO-101) is an ATP-competitive oral, selective inhibitor of the tropomyosin-related kinase (TRK) family receptors, with low nanomolar 50% inhibitory concentrations against all three isoforms (TRKA, B, and C). TrkA TrkB TrkC
Larotrectinib (LOXO-101) is an ATP-competitive oral inhibitor of the tropomyosin-related kinase (TRK) family of receptor kinases (TRKA, B, and C), with low nanomolar 50% inhibitory concentrations against all three isoforms, and 1,000-fold or greater selectivity relative to other kinases[1][2]. Measurement of proliferation following treatment with Larotrectinib (LOXO-101) demonstrates a dose-dependent inhibition of cell proliferation in all three cell lines. The IC50 is less than 100 nM for CUTO-3.29 and less than 10 nM for KM12 and MO-91 consistent with the known potency of this drug for the TRK kinase family[3].
In rat and monkey studies, Larotrectinib (LOXO-101) demonstrates 33-100% oral bioavailability and 60-65% plasma protein binding. It has low brain penetration, and is well tolerated in 28 day (d) GLP toxicology studies. A single dose (30 mg/kg) of Larotrectinib (LOXO-101) reduces tyrosine phosphorylation of TRKA and downstream signal transduction (pERK) in the tumor >80%[1]. Athymic nude mice injected with KM12 cells are treated with Larotrectinib (LOXO-101) orally daily for 2 weeks. Dose-dependent tumor inhibition is observed demonstrating the ability of this selective compound to inhibit tumor growth in vivo[4]. Larotrectinib (LOXO-101) (200mg/kg/day p.o for six weeks) reduces leukemic infiltration to undetectable levels in the bone marrow and spleen compared to vehicle-treated mice. Mice treated with Larotrectinib (LOXO-101) are still alive and leukemia-free four weeks after the cessation of treatment, as determined by Xenogen imaging[5].
References:
[1]. Karyn Bouhana, et al. LOXO-101, a pan TRK inhibitor, For The Treatment Of TRK-driven Cancers.
[2]. Nagasubramanian R, et al. Infantile Fibrosarcoma With NTRK3-ETV6 Fusion Successfully Treated With the Tropomyosin-Related Kinase Inhibitor LOXO-101. Pediatr Blood Cancer. 2016 Aug;63(8):1468-70.
[3]. Doebele RC, et al. An Oncogenic NTRK Fusion in a Patient with Soft-Tissue Sarcoma with Response to the Tropomyosin-Related Kinase Inhibitor LOXO-101. Cancer Discov. 2015 Oct;5(10):1049-57.
[4]. Doebele RC, et al. An Oncogenic NTRK Fusion in a Patient with Soft-Tissue Sarcoma with Response to the Tropomyosin-Related Kinase Inhibitor LOXO-101. Cancer Discov. 2015 Oct;5(10):1049-57.
[5]. Kathryn G, et al. Genetic Modeling and Therapeutic Targeting of ETV6-NTRK3 with Loxo-101in Acute Lymphoblastic Leukemia. Blood 2016 128:278.
Average Rating: 5 (Based on Reviews and 34 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















