Ginkgolide J (Synonyms: BN 52024) |
| Catalog No.GC38027 |
Products are for research use only. Not for human use. We do not sell to patients.
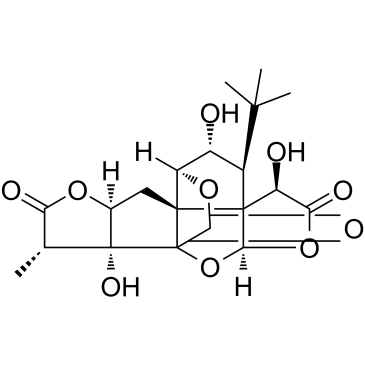
Cas No.: 107438-79-9
Sample solution is provided at 25 µL, 10mM.
Ginkgolide J is a main constituent of the non-flavone fraction of Ginkgo biloba with an IC50 range of 12-54 µM, has neuroprotective and anti neuronal apoptotic ability[1][2].
Ginkgolide J (100 μM) treatment reduces the apoptotic damage induced by serum deprivation (24h) or treatment with Staurosporine (200 nM, 24h) in cultured chick embryonic neurons[1].
[1]. Ahlemeyer B, et al. Pharmacological studies supporting the therapeutic use of Ginkgo biloba extract for Alzheimer's disease. Pharmacopsychiatry. 2003 Jun; 36 Suppl 1:S8-14. [2]. Sylvia Pietri, et al. Synthesis and Biological Studies of a New Ginkgolide C Derivative: Evidence That the Cardioprotective Effect of Ginkgolides Is Unrelated to PAF Inhibition. DRUG DEVELOPMENT RESEARCH. 2001.54:191-201.
Average Rating: 5 (Based on Reviews and 5 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















