Gefapixant (Synonyms: AF219; MK-7264)) |
| Catalog No.GC19422 |
Gefapixant is a P2X3 receptor antagonist
Products are for research use only. Not for human use. We do not sell to patients.
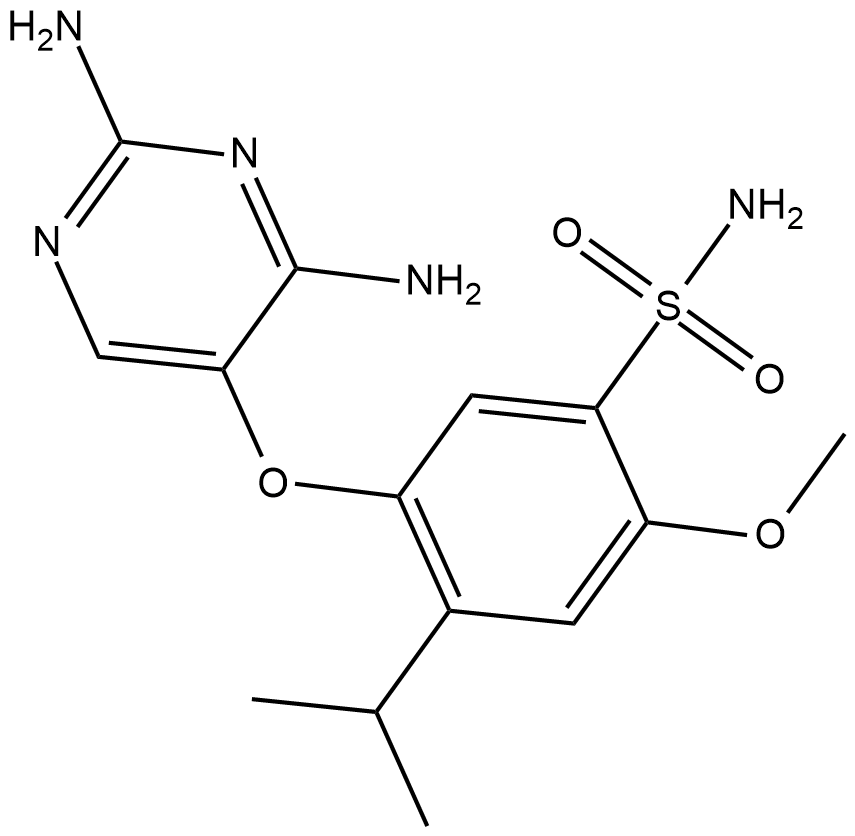
Cas No.: 1015787-98-0
Sample solution is provided at 25 µL, 10mM.
Gefapixant is an orally active P2X3 receptor (P2X3R) antagonist with IC 50s of ~30 nM versus recombinant hP2X3 homotrimers and 100-250 nM at hP2X2/3 heterotrimeric receptors.
The aryloxy-pyrimidinediamine, Gefapixant (AF-219) is an orally active small molecule antagonist at human P2X3-containing receptors. The IC50 of Gefapixant has been reported as ~30 nM versus recombinant hP2X3 homotrimers and 100-250 nM at hP2X2/3 heterotrimeric receptors, potencies very similar to those reported for recombinant rat receptors, and it displays no inhibitory impact on any non-P2X3 subunit containing receptors (IC50 values>>10,000 nM at recombinant homotrimeric hP2X1, hP2X2, hP2X4, rP2X5 and hP2X7 channels)[1].
In an adjuvant-induced rthritis model in rat (7d following intraplantar administration of complete Freund's adjuvant), AF-353 produces dose-dependent antihyperalgesia in weight-bearing asymmetry and von Frey filament mechanical tests; magnitude of effect is compared with that of the NSAID naproxen. In a rat model of knee osteoarthritis (14d following intra-articular administration of monoiodoacetate), Gefapixant (7d bid, orally; right) attenuates the weight bearing laterality with complete reversal of apparent hyperalgesia at the two higher doses[2].
References:
[1]. Anthony P. Ford, et al. The therapeutic promise of ATP antagonism at P2X3 receptors in respiratory and urological disorders. Front Cell Neurosci. 2013; 7: 267.
[2]. Ford AP, In pursuit of P2X3 antagonists: novel therapeutics for chronic pain and afferent sensitization. Purinergic Signal. 2012 Feb;8(Suppl 1):3-26.
Average Rating: 5 (Based on Reviews and 11 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















