Glycopyrrolate (Synonyms: AHR 504, NSC 250836, NSC 251251, NSC 251252) |
| Catalog No.GC15029 |
Muscarinic competitive antagonist
Products are for research use only. Not for human use. We do not sell to patients.
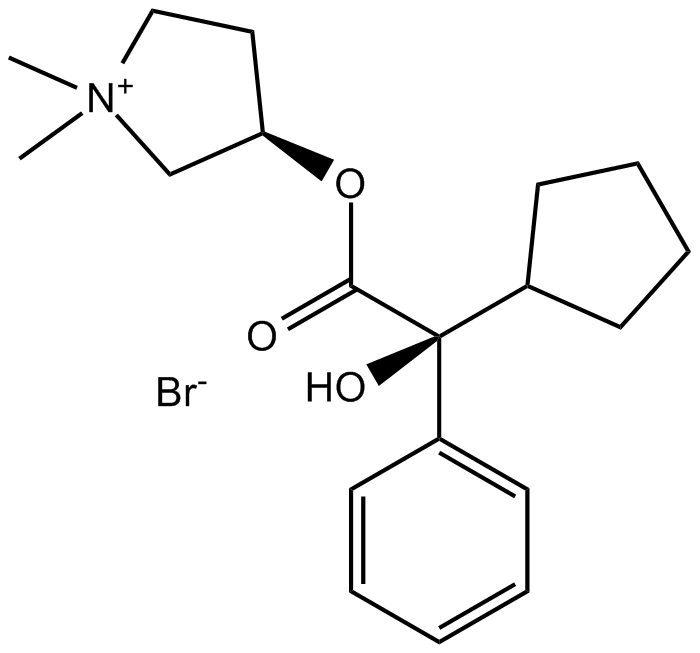
Cas No.: 596-51-0
Sample solution is provided at 25 µL, 10mM.
Glycopyrrolate (Glycopyrronium bromide) is a muscarinic competitive antagonist used as an antispasmodic.IC50 Value:Target: mAChR (Muscarinic acetylcholine receptor M1)in vitro: Glycopyrrolate showed no selectivity in its binding to the M1-M3 receptors. Kinetics studies, however, showed that glycopyrrolate dissociates slowly from HASM muscarinic receptors (60% protection against [3H]-NMS binding at 30 nM) compared to ipratropium bromide [1].in vivo: Glycopyrrolate (1 mg) tablets were then administered, starting with one tablet daily the third week and increasing the daily dose by one tablet per week until a maximum of four tablets during week six and 4 days of week seven when the daily dose was reduced to two tablets for 3 days. glycopyrrolate can be given in controlled doses provided that an adequate medical assessment has been undertaken [2]. Glycopyrrolate has a slow and erratic absorption from the gastrointestinal system, but even low plasma levels are associated with a distinct and long-lasting antisialogic effect [3]. Oral glycopyrrolate is emerging as a potential second-line treatment option, but experience with safety, efficacy, and dosing is especially limited in children [4]. phase III study, 52.3% of glycopyrrolate oral solution recipients (aged 3-18 years; n = 137) had an mTDS response (primary endpoint); the response rate was consistently above 50% at all 4-weekly timepoints, aside from the first assessment at week 4 (40.3%). In general, glycopyrrolate oral solution was well tolerated in clinical trials. The majority of adverse events were within expectations as characteristic anticholinergic outcomes [5].Toxicity: Side effects include dry mouth, difficult urinating, heachaches, diarrhea and constipation. The medication also induces drowsiness or blurred vision. LD50=709 mg/kg (rat, oral).
References:
[1]. Haddad, E.B., et al., Pharmacological characterization of the muscarinic receptor antagonist, glycopyrrolate, in human and guinea-pig airways. Br J Pharmacol, 1999. 127(2): p. 413-20.
[2]. Neverlien, P.O., et al., Glycopyrrolate treatment of drooling in an adult male patient with cerebral palsy. Clin Exp Pharmacol Physiol, 2000. 27(4): p. 320-2.
[3]. Olsen, A.K. and P. Sjogren, Oral glycopyrrolate alleviates drooling in a patient with tongue cancer. J Pain Symptom Manage, 1999. 18(4): p. 300-2.
[4]. Kumar, M.G., et al., Oral Glycopyrrolate for Refractory Pediatric and Adolescent Hyperhidrosis. Pediatr Dermatol, 2013.
[5]. Garnock-Jones, K.P., Glycopyrrolate oral solution: for chronic, severe drooling in pediatric patients with neurologic conditions. Paediatr Drugs, 2012. 14(4): p. 263-9.
Average Rating: 5 (Based on Reviews and 13 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















