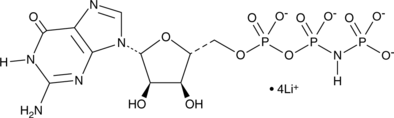
Guanylyl Imidodiphosphate (lithium salt) (Synonyms: Gpp(NH)p lithium) |
| Catalog No.GC43798 |
Guanylyl imidodiphosphate (Gpp(NH)p) lithium, a non-hydrolyzable GTP analogue, increases adenylate cyclase activity.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 64564-03-0
Sample solution is provided at 25 µL, 10mM.
Guanylyl imidodiphosphate is a nonhydrolyzable analog of GTP that can bind to and irreversibly activate G proteins in the presence of Mg2+.[1] This nucleotide is a potent stimulator of adenylate cyclase.[2] Guanylyl imidodiphosphate is used in studies of protein synthesis since the process of GTP binding, hydrolysis, and release is essential for the initiation of protein translocation across the endoplasmic reticulum.[3]
Reference:
[1]. Terry, B.J., and Purich, D.L. Assembly and disassembly properties of microtubules formed in the presence of GTP, 5'-guanylyl imidodiphosphate, and 5'-guanylyl methylenediphosphate. The Journal of Biological Chemisty 255(21), 10532-10536 (1980).
[2]. Lefkowitz, R.J., and Caron, M.G. Characteristics of 5'-guanylyl imidodiphosphate-activated adenylate cyclase. The Journal of Biological Chemisty 250(12), 4418-4422 (1975).
[3]. Rapoport, T.A. Transport of proteins across the endoplasmic reticulum membrane. Science 258(5084), 931-936 (1992).
Review for Guanylyl Imidodiphosphate (lithium salt)
Average Rating: 5 (Based on Reviews and 6 reference(s) in Google Scholar.)
Review for Guanylyl Imidodiphosphate (lithium salt)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















