Hydroxyfasudil hydrochloride |
| Catalog No.GC10338 |
A ROCK inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
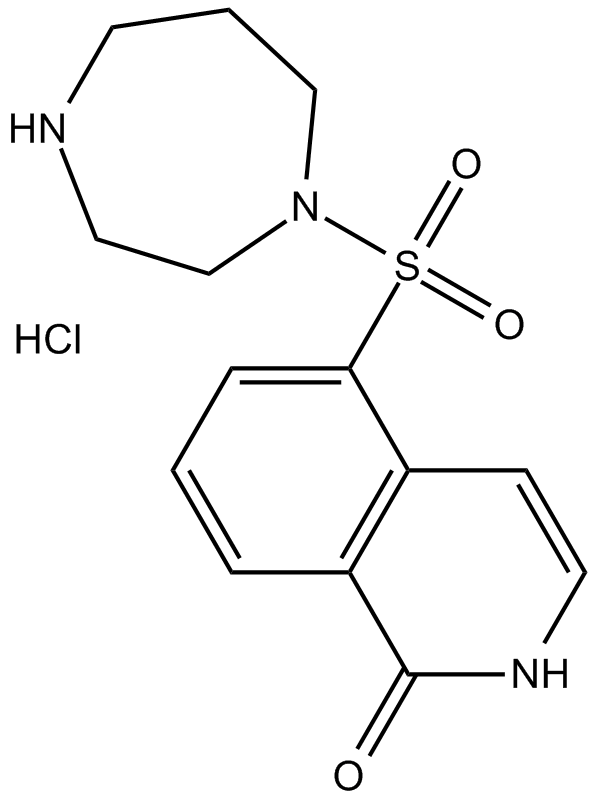
Cas No.: 155558-32-0
Sample solution is provided at 25 µL, 10mM.
Description:
IC50 Value: 0.12 uM (ROCK1); 0.17 uM (ROCK2) [1]
Hydroxyfasudil, metabolite of Fasudil, is a potent Rho-kinase inhibitor and vasodilator.
in vitro: Fasudil (1-10 μM) and hydroxyfasudil (0.3-10 μM) significantly prevented endothelin-induced cardiomyocyte hypertrophy [2]. Hydroxyfasudil significantly attenuated serotonin (IC)-induced vasoconstriction of SA (-7 +/- 1% vs. 2 +/- 1%, p < 0.01). Coronary I/R significantly impaired coronary vasodilation to acetylcholine after I/R (SA, p < 0.05; and A, p < 0.01 vs. before I/R) and L-NMMA further reduced the vasodilation, whereas hydroxyfasudil completely preserved the responses.
in vivo: Treatment with hydroxyfasudil significantly improved bladder intercontraction intervals. Rats treated with hydroxyfasudil also showed a significant reduction of histopathological features associated with cystitis [3]. Twelve-week-old male SHRs were treated with hydroxyfasudil (3 or 10 mg/kg, i.p.) once a day for 6 weeks. Treatment with hydroxyfasudil significantly improved the decreased penile cGMP concentrations, the increased Rho kinase activities, the increased norepinephrine-induced contractions, and the decreased acetylcholine-induced relaxation in a dose-dependent manner [4].
Toxicity: The proportion of patients with good clinical outcome was 74.5% (41/55) in the fasudil group and 61.7% (37/60) in the nimodipine group. There were no serious adverse events reported in the fasudil group [5].
Clinical trial: N/A
Review for Hydroxyfasudil hydrochloride
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
Review for Hydroxyfasudil hydrochloride
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















