Hydroxytacrine (maleate) (Synonyms: HP 029,1-Hydroxytacrine,P 83-6029A,Velnacrine) |
| Catalog No.GC15604 |
anticholinesterase activity
Products are for research use only. Not for human use. We do not sell to patients.
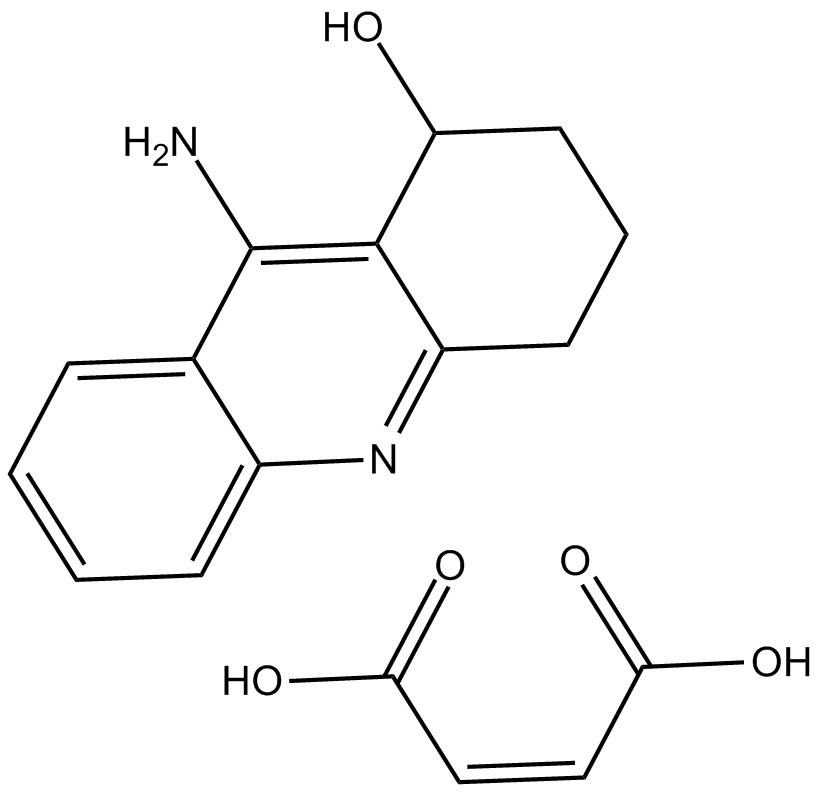
Cas No.: 118909-22-1
Sample solution is provided at 25 µL, 10mM.
Hydroxytacrine maleate, a bioactive monohydroxylated metabolite of cholinesterase inhibitor, is a potential Alzheimer's therapeutic of low toxicity [1]. Hydroxytacrine maleate exhibited biochemical and pharmacological profile similar to tacrine (THA) except that the far less liver toxicity in humans. The prolonged use of tacrine has been associated with liver toxicity[1, 2].
Hydroxytacrine maleate is a parasympathomimetic and a centrally acting cholinesterase inhibitor (anticholinesterase). As the first cholinesterase inhibitor approved for the treatment of AD, tacrine was marketed under the trade name Cognex [3]. Through hydroxylation of benzylic carbon by CYP450 in the liver, tacrine has been metabolized into metabolite 1-hydroxy-tacrine (velnacrine) [4]. It has also been shown that maleate is an inhibitor of AChE [5].
References:
[1]. D. Muoz-Torrero. Acetylcholinesterase inhibitors as disease-modifying therapies for Alzheimer’s disease. Curr. Med. Chem. 15, 2433-2455 (2008).
[2]. E. Giacobini. Cholinesterase inhibitors for Alzheimer’s disease therapy: From tacrine to future applications. Neurochemistry International 32, 413-419(1998).
[3]. Birks J S. Cholinesterase inhibitors for Alzheimer's disease[J]. The Cochrane Library, 2006.
[4]. Peng J Z, Remmel R P, Sawchuk R J. Inhibition of murine cytochrome P4501A by tacrine: in vitro studies[J]. Drug metabolism and disposition, 2004, 32(8): 805-812.
[5]. Acetylcholine and choline effects on erythrocyte nitrite and nitrate levels.
Average Rating: 5 (Based on Reviews and 33 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















